MECHANISM OF DISEASE
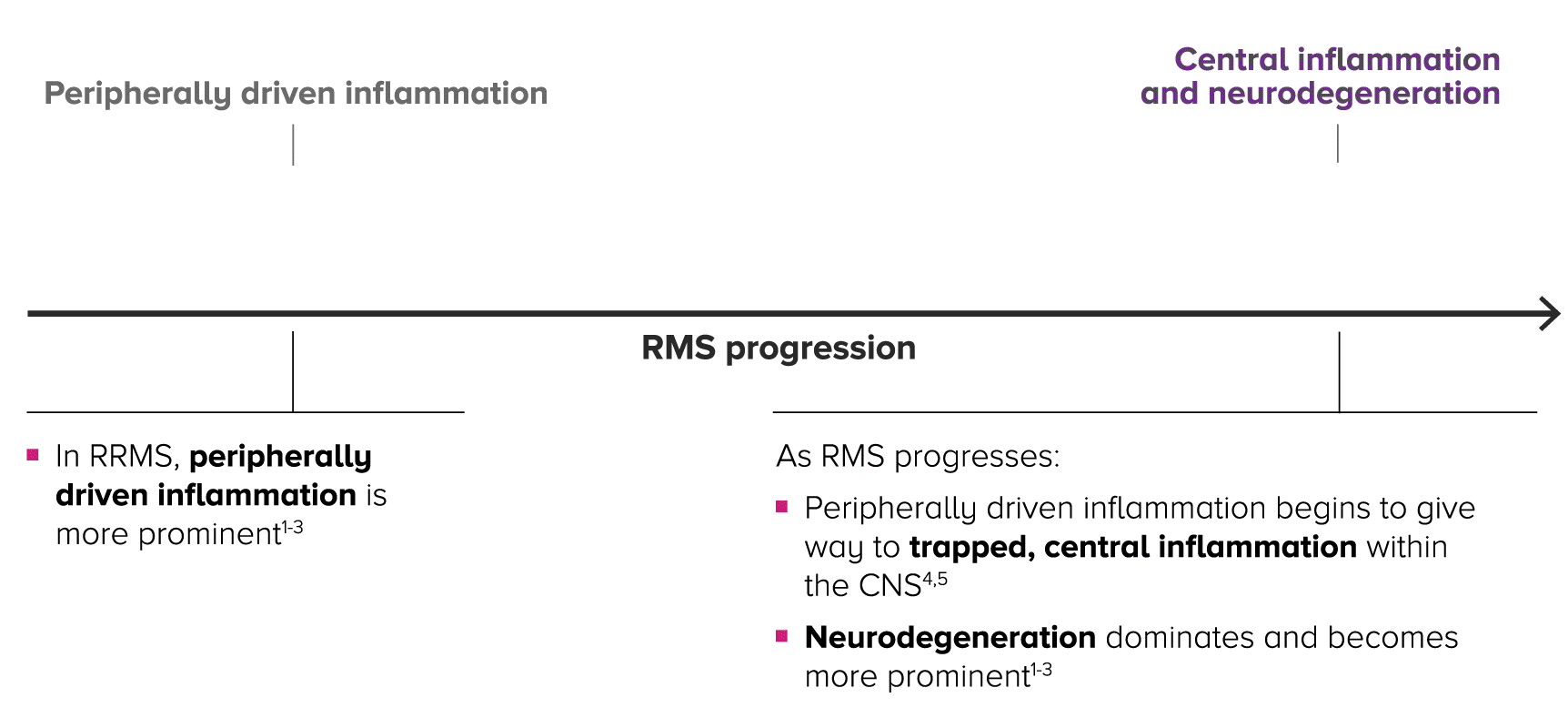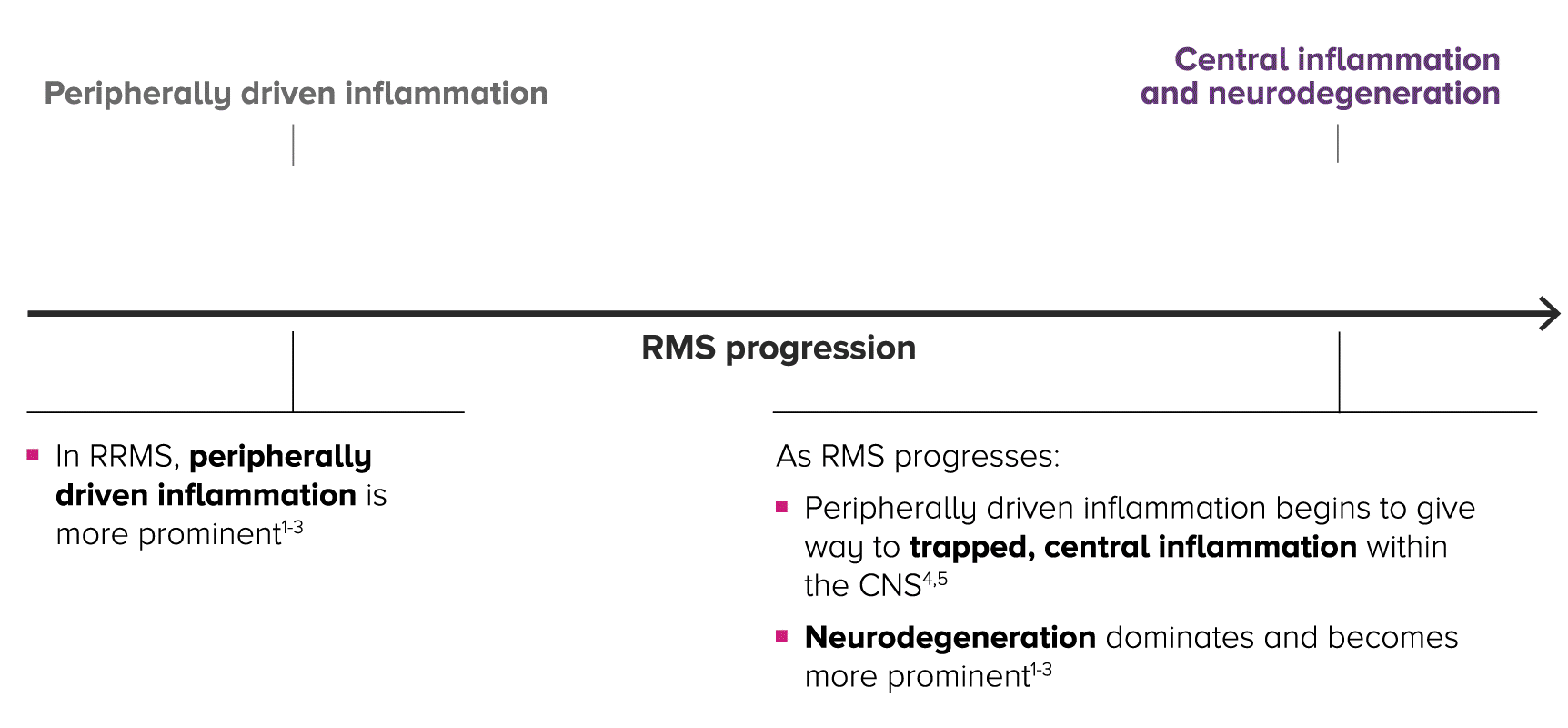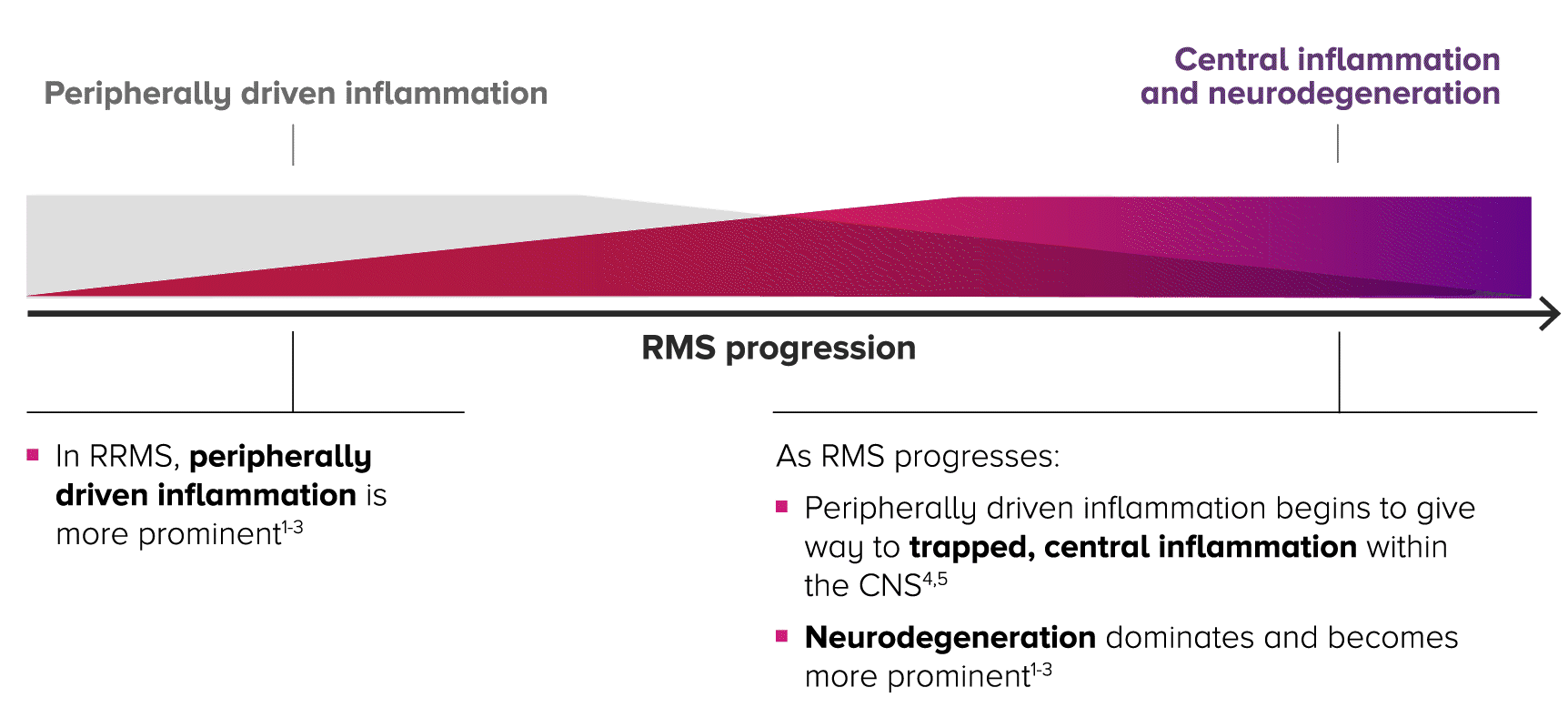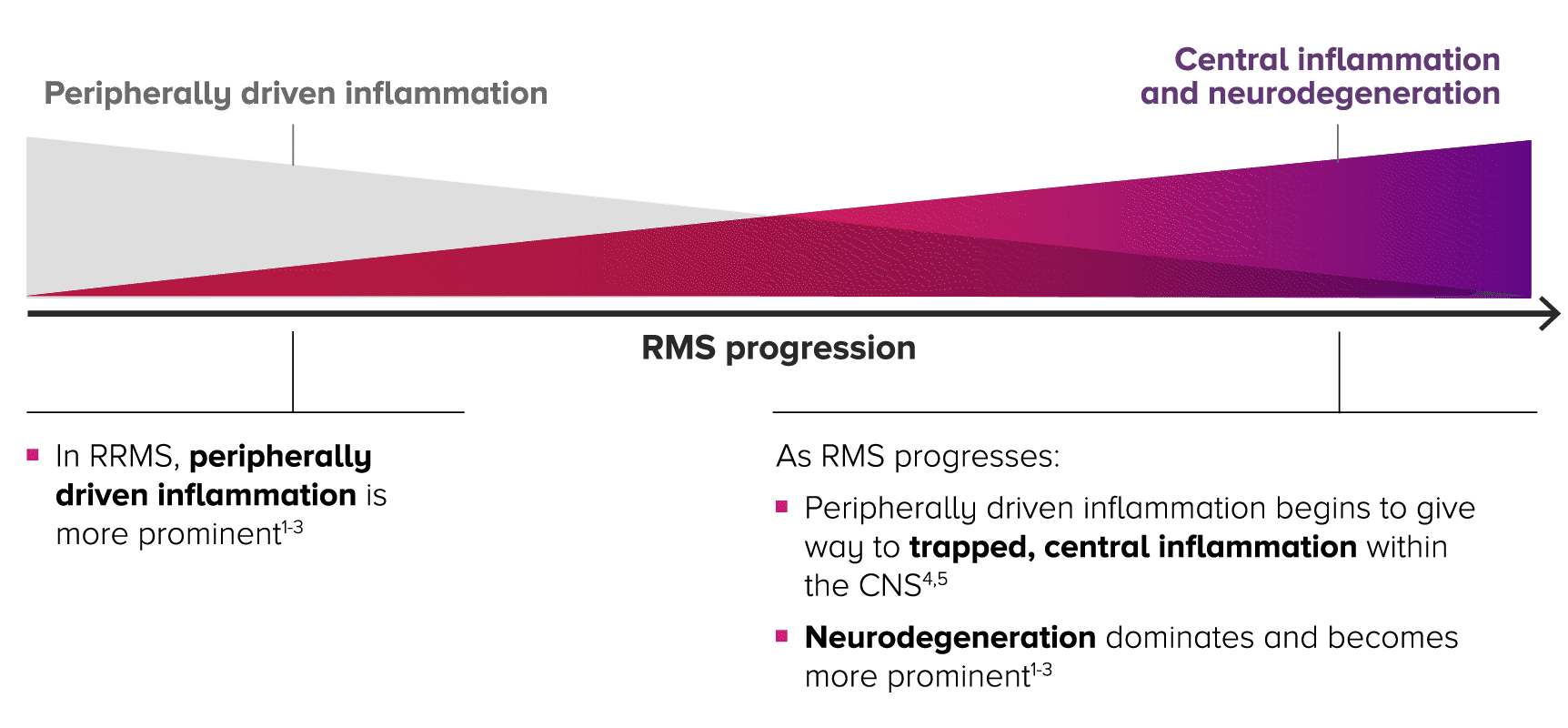
IN RELAPSING MS PROGRESSION, THERE’S AN INVERSE RELATIONSHIP BETWEEN INFLAMMATION AND NEURODEGENERATION1-4
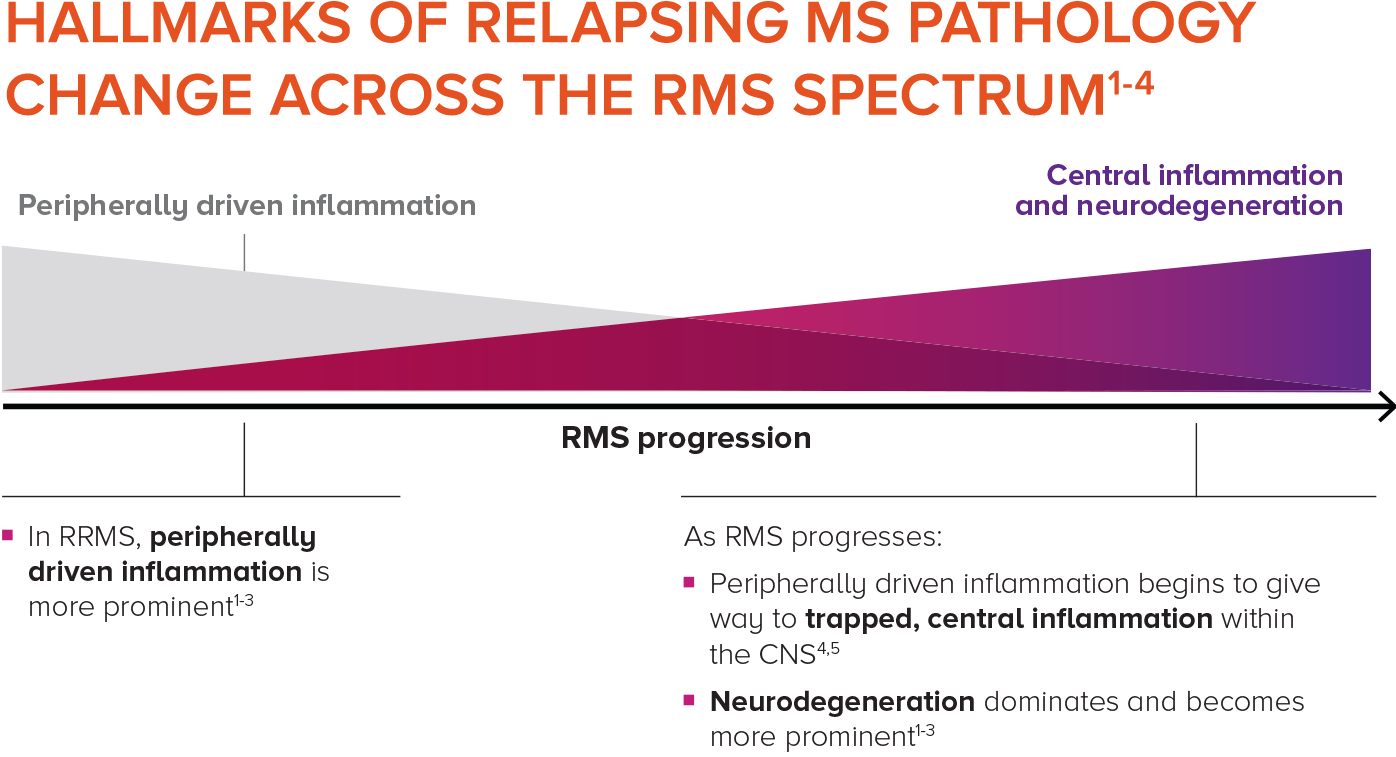
Relapsing MS is progressive and changes over time, with central inflammation contributing to cumulative, irreversible disability2-4
Relapsing MS is progressive and changes over time, with central inflammation contributing to cumulative, irreversible disability2-4
As patients progress in RMS, peripherally driven inflammation and central inflammation are at play—with central inflammation and neurodegeneration becoming more prominent over time within the CNS.4,5
HALLMARKS OF RELAPSING MS PATHOLOGY CHANGE ACROSS THE RMS SPECTRUM1-4
LOOK BEYOND RELAPSES
Patients with more progressed RMS, including active SPMS, typically have fewer relapses and/or diminishing MRI activity but are showing signs of progression characterized by cumulative disability, including cognitive impairment. Look further for early identification of progression—monitor for both physical and cognitive changes.6-9
LOOK BEYOND RELAPSES
Patients with more progressed RMS, including active SPMS, typically have fewer relapses and/or diminishing MRI activity but are showing signs of progression characterized by cumulative disability, including cognitive impairment. Look further for early identification of progression—monitor for both physical and cognitive changes.6-9
MECHANISM OF ACTION
THE DUAL MOA OF MAYZENT® TARGETS S1P1,5—2 KEY
RECEPTORS THOUGHT TO PLAY A ROLE IN RELAPSING MS
INFLAMMATION AND NEURODEGENERATION10-14
THE DUAL MOA OF MAYZENT® TARGETS S1P1,5—2 KEY RECEPTORS THOUGHT TO PLAY A ROLE IN RELAPSING MS INFLAMMATION AND NEURODEGENERATION10-14
MAYZENT is an oral, selective S1P receptor modulator for progressing patients along the relapsing MS continuum10
MAYZENT is an oral, selective S1P receptor modulator for progressing patients along the relapsing MS continuum10
SEE MAYZENT IN MOTION IN:
SEE MAYZENT IN MOTION IN:
BEAUTIFUL SCIENCE: THE DUAL MOA OF MAYZENT
BEAUTIFUL SCIENCE: THE DUAL MOA OF MAYZENT
THE PROPOSED DUAL MOA OF MAYZENT
THE PROPOSED DUAL MOA OF MAYZENT
WORKS IN THE PERIPHERY to limit active lymphocytes from leaving the lymph nodes, which may reduce lymphocyte migration into the CNS10,15
-
Lymphocytes are stored, not destroyed, in the lymph nodes; upon discontinuation, blood lymphocyte counts rapidly recover within 10 days10,15*†
WORKS CENTRALLY by crossing the blood-brain barrier into the CNS.10,15,16
Based on animal studies, siponimod readily crosses the blood-brain barrier and enters the CNS.10,15,16
WORKS IN THE PERIPHERY to limit active lymphocytes from leaving the lymph nodes, which may reduce lymphocyte migration into the CNS10,15
-
Lymphocytes are stored, not destroyed, in the lymph nodes; upon discontinuation, blood lymphocyte counts rapidly recover within 10 days10,15*†
WORKS CENTRALLY by crossing the blood-brain barrier into the CNS.10,15,16
Based on animal studies, siponimod readily crosses the blood-brain barrier and enters the CNS.10,15,16
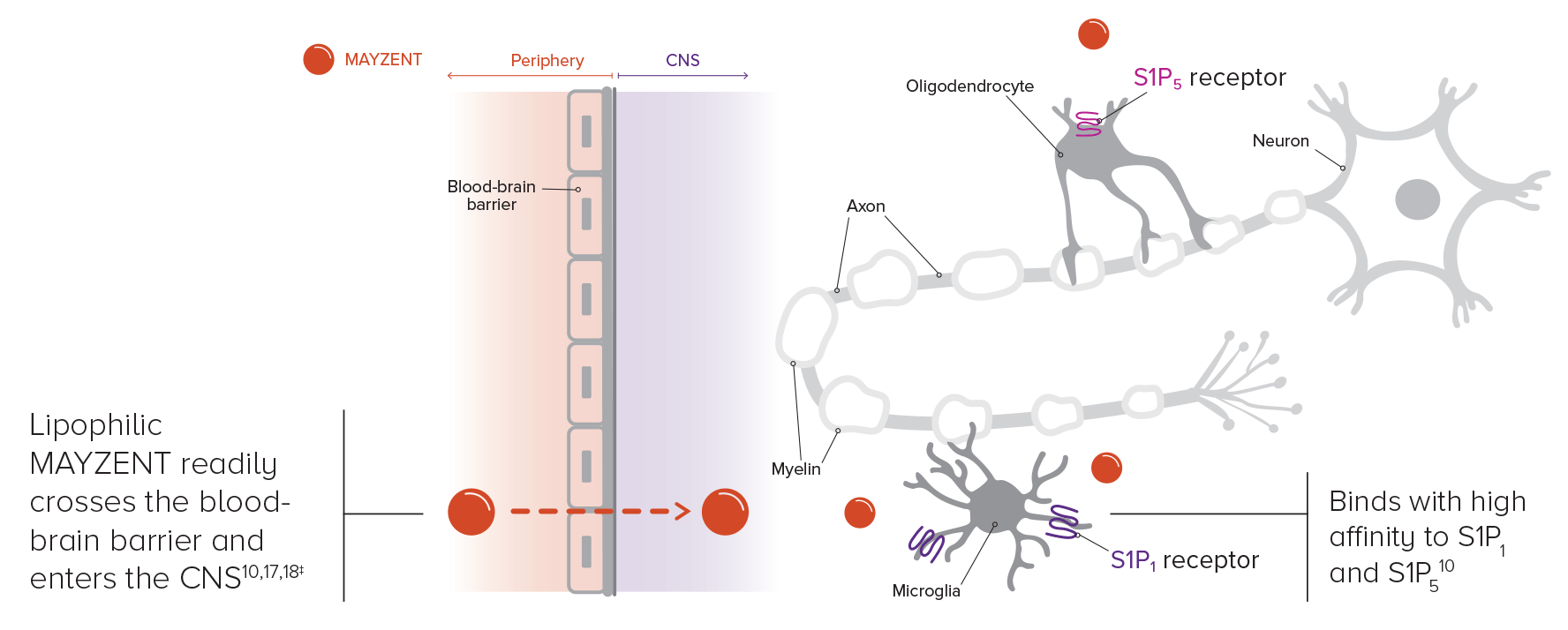

The mechanism by which siponimod exerts therapeutic effects on MS is unknown but may involve reduction of lymphocytes in the CNS.10
BELIEVED TO WORK IN 2 PLACES: PERIPHERY AND CNS10,15,16,19

GET PATIENTS STARTED ON MAYZENT®
CNS, central nervous system; MOA, mechanism of action; MOD, mechanism of disease; MRI, magnetic resonance imaging; MS, multiple sclerosis; RMS, relapsing MS; RRMS, relapsing-remitting MS; S1P, sphingosine 1-phosphate; SPMS, secondary progressive MS.
*In 90% of patients.10
†After stopping MAYZENT treatment, residual lowering of peripheral lymphocyte count may persist for up to 3-4 weeks after the last dose.10
‡Based on animal studies.10,15,16
References: 1. Hauser SL, Oksenberg JR. The neurobiology of multiple sclerosis: genes, inflammation, and neurodegeneration. Neuron. 2006;52(1):61-76. 2. Ziemssen T, Derfuss T, de Stefano N, et al. Optimizing treatment success in multiple sclerosis. J Neurol. 2016;263(6):1053-1065. 3. Dendrou CA, Fugger L, Friese MA. Immunopathology of multiple sclerosis. Nat Rev Immunol. 2015;15(9)545-558. doi:10.1038/nri3871. 4. Kutzelnigg A, Lucchinetti CF, Stadelmann C, et al. Cortical demyelination and diffuse white matter injury in multiple sclerosis. Brain. 2005;128(Pt 11):2705-2712. 5. Lassmann H, van Horssen J, Mahad D. Progressive multiple sclerosis: pathology and pathogenesis. Nat Rev Neurol. 2012;8(11):647-656. 6. Fox RJ, Cohen JA. Multiple sclerosis: The importance of early recognition and treatment. Cleve Clin J Med. 2001;68(2):157-171. 7. Ontaneda D, Thompson AJ, Fox RJ, Cohen JA. Progressive multiple sclerosis: prospects for disease therapy, repair, and restoration of function. Lancet. 2017;389(10076):1357-1366. 8. Kurtzke JF. Rating neurologic impairment in multiple sclerosis: an expanded disability status scale (EDSS). Neurology. 1983;33(11):1444-1452. 9. Brochet B, Ruet A. Cognitive impairment in multiple sclerosis with regards to disease duration and clinical phenotypes. Front Neurol. 2019;10:261. doi:10.3389/fneur.2019.0026 10. Mayzent [prescribing information]. East Hanover, NJ: Novartis Pharmaceuticals Corp. 11. O’Sullivan C, Schubart A, Mir AK, Dev KK. The dual S1PR1/S1PR5 drug BAF312 (siponimod) attenuates demyelination in organotypic slice cultures. J Neuroinflammation. 2016;13:31. 12. Gergely P, Nuesslein-Hildesheim B, Guerini D, et al. The selective sphingosine 1-phosphate receptor modulator BAF312 redirects lymphocyte distribution and has species-specific effects on heart rate. Br J Pharmacol. 2012;167(5):1035-1037. 13. Mannioui A, Vauzanges Q, Fini JB, et al. The Xenopus tadpole: an in vivo model to screen drugs favoring remyelination. Mult Scler. 2018;24(11):1421-1432. 14. Choi JW, Chun J. Lysophospholipids and their receptors in the central nervous system. Biochim Biophys Acta. 2013;1831(1):20-32. 15. Gentile A, Musella A, Bullitta S, et al. Siponimod (BAF312) prevents synaptic neurodegeneration in experimental multiple sclerosis. J Neuroinflammation. 2016;13(1):207. 16. Bigaud M, Rudolph B, Briard E, Beerli C, Schubart A, Gardin A. Siponimod penetrates, distributes and acts on the central nervous system: translational insights. Neurology. 2020;94(suppl 15):3973. American Academy of Neurology abstract 3973. 17. Briard E, Rudolph B, Desravaud S, Krauser JA, Auberson YP. MS565: a SPECT tracer for evaluating the brain penetration of BAF312 (siponimod). ChemMedChem. 2015;10(6):1008-1018. 18. Behrangi N, Fischbach F, Kipp M. Mechanism of siponimod: anti-inflammatory and neuroprotective mode of action. Cells. 2019;8(24):1-11. 19. Data on file. Dual mode of action of siponimod in secondary progressive multiple sclerosis: a hypothesis based on the relevance of pharmacological properties. Novartis Pharmaceuticals Corp; September 2020.