EXPAND STUDIED A BROAD RANGE OF PATIENTS WITH MODERATE-TO-ADVANCED DISABILITY—INCLUDING PATIENTS PREVIOUSLY TREATED WITH A DMT1
EXPAND STUDIED A BROAD RANGE OF PATIENTS WITH MODERATE-TO-ADVANCED DISABILITY—INCLUDING PATIENTS PREVIOUSLY TREATED WITH A DMT1
MAYZENT® was evaluated in EXPAND—the largest Phase III study of SPMS patients to date (N=1651)2
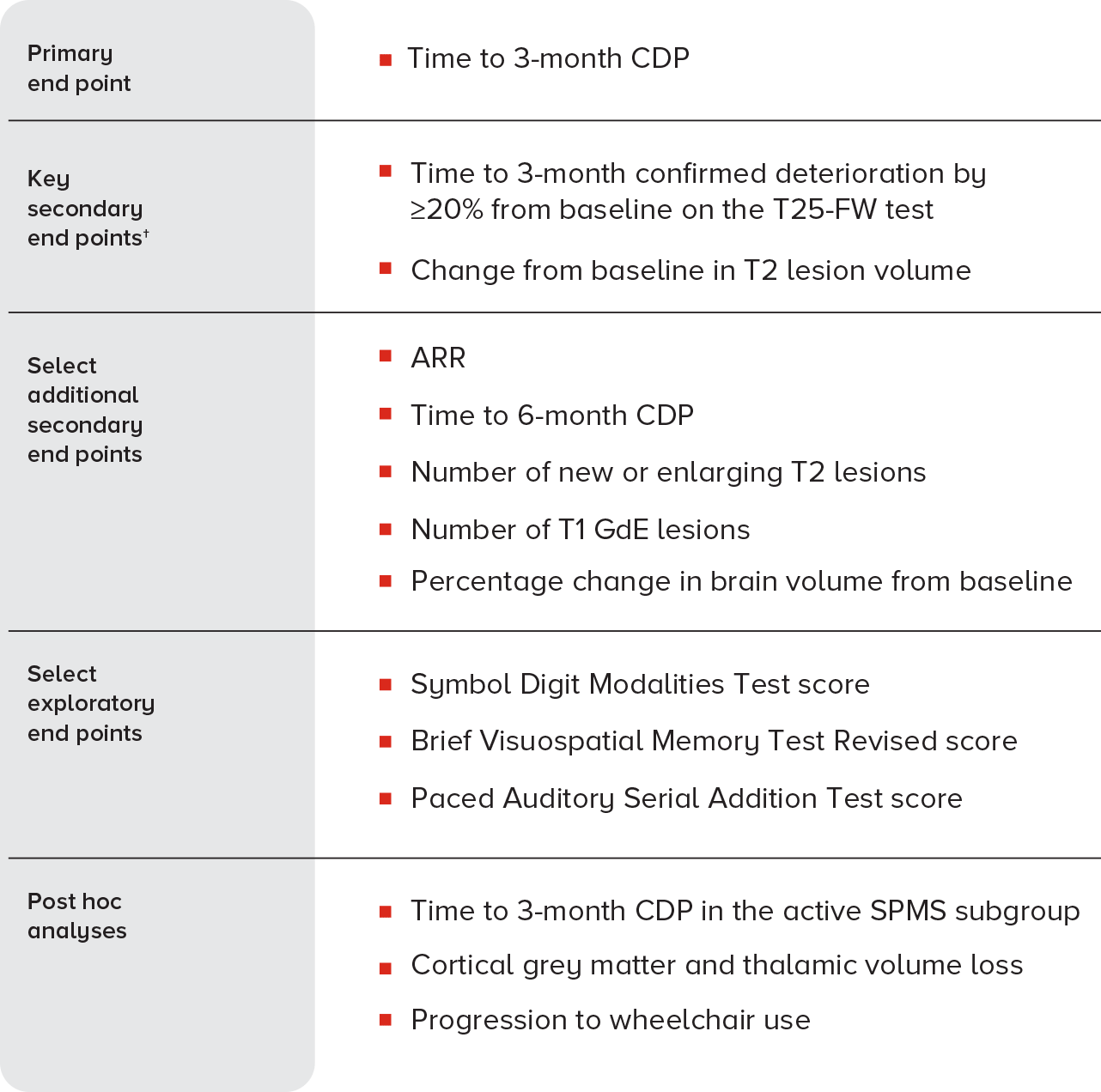
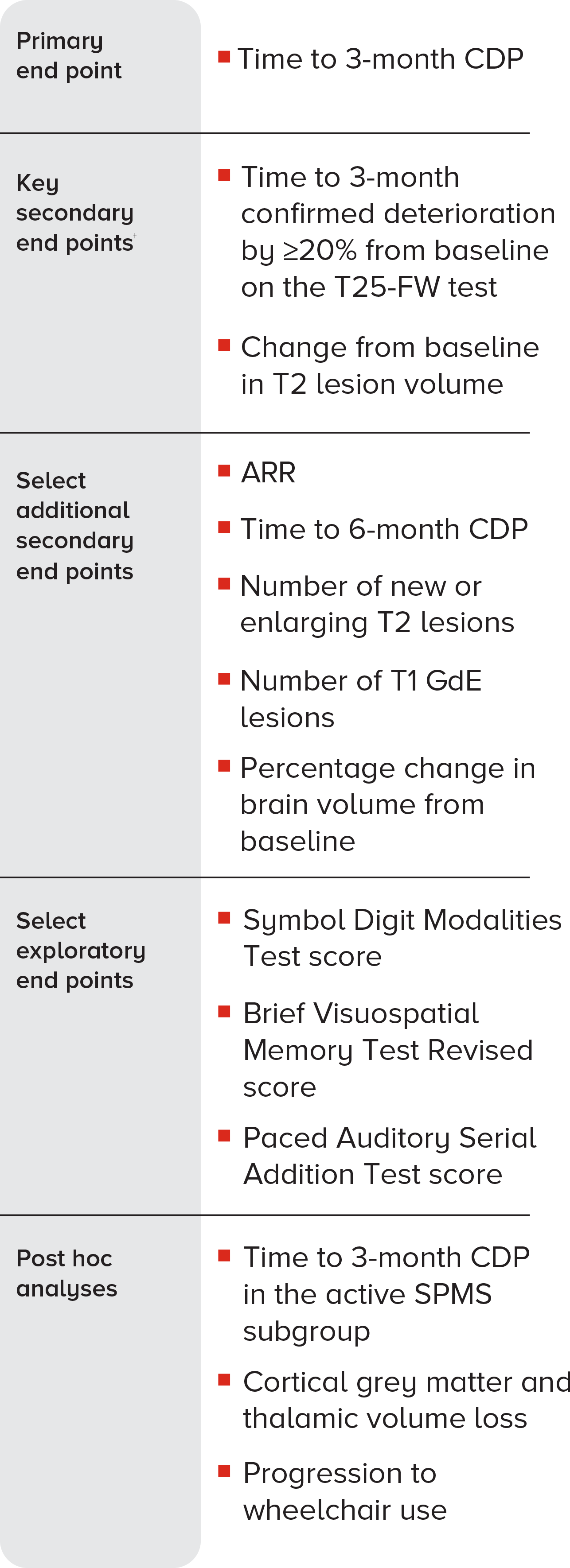
TRIAL DESIGN1,3

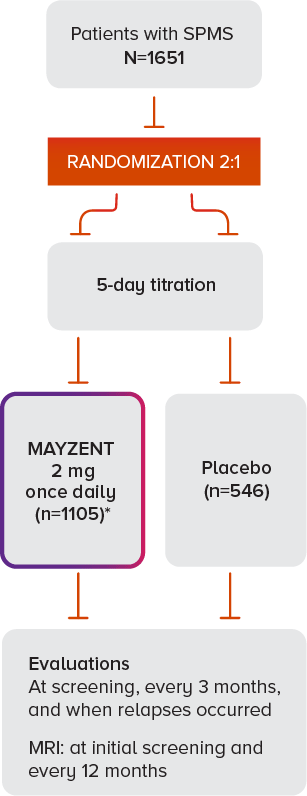
*Median drug exposure of 18 months.
BASELINE PATIENT CHARACTERISTICS FOR PATIENTS ON MAYZENT1
HOW TO ASSESS EDSS SCORESGET PATIENTS STARTED ON MAYZENT®
ARR, annualized relapse rate; CDP, confirmed disability progression; DMT, disease-modifying therapy; EDSS, Expanded Disability Status Scale; GdE, gadolinium-enhancing; MRI, magnetic resonance imaging; MS, multiple sclerosis; SPMS, secondary progressive MS; T25-FW, timed 25-foot walk.
†In EXPAND, a prespecified hierarchical analysis consisted of the primary end point and 2 key secondary end points. The T25-FW test key end point was not significant; therefore, the T2 lesion volume key secondary end point was considered nominal. The remaining end points were not corrected for multiple comparisons.1,3
References: 1. Kappos L, Bar-Or A, Cree BAC, et al; for the EXPAND Clinical Investigators. Siponimod versus placebo in secondary progressive multiple sclerosis (EXPAND): a double-blind, randomised, phase 3 study. Lancet. 2018;391(10127):1263-1273. 2. Data on file. US National Library of Medicine. ClinicalTrials.gov. SPMS Clinical Trial Search Results. Accessed February 11, 2022. 3. Mayzent [prescribing information]. East Hanover, NJ: Novartis Pharmaceuticals Corp. 4. Data on file. Impact of Siponimod on Cognition in Patients With Secondary Progressive Multiple Sclerosis: Results From Phase 3 EXPAND Study. Novartis Pharmaceuticals Corp; 2017. 5. Data on file. Efficacy of Siponimod in Secondary Progressive Multiple Sclerosis Patients With Active Disease: The EXPAND Study Subgroup Analysis. Novartis Pharmaceuticals Corp; August 2019. 6. Data on file. Effect of Siponimod on Cortical Grey Matter and Thalamic Volume in Patients With Secondary Progressive Multiple Sclerosis—Results of the EXPAND Study. Novartis Pharmaceuticals Corp; May 2020. 7. Data on file. Data Analysis Report Time to Wheelchair. Novartis Pharmaceuticals Corp; September 2019.