MAYZENT® WAS PROVEN TO DELAY DISABILITY PROGRESSION IN EXPAND 1
MAYZENT® WAS PROVEN TO DELAY DISABILITY PROGRESSION IN EXPAND 1


CORE STUDY
DELAYED DISABILITY PROGRESSION: CORE STUDY1POST HOC ACTIVE SPMS SUBGROUP ANALYSIS4
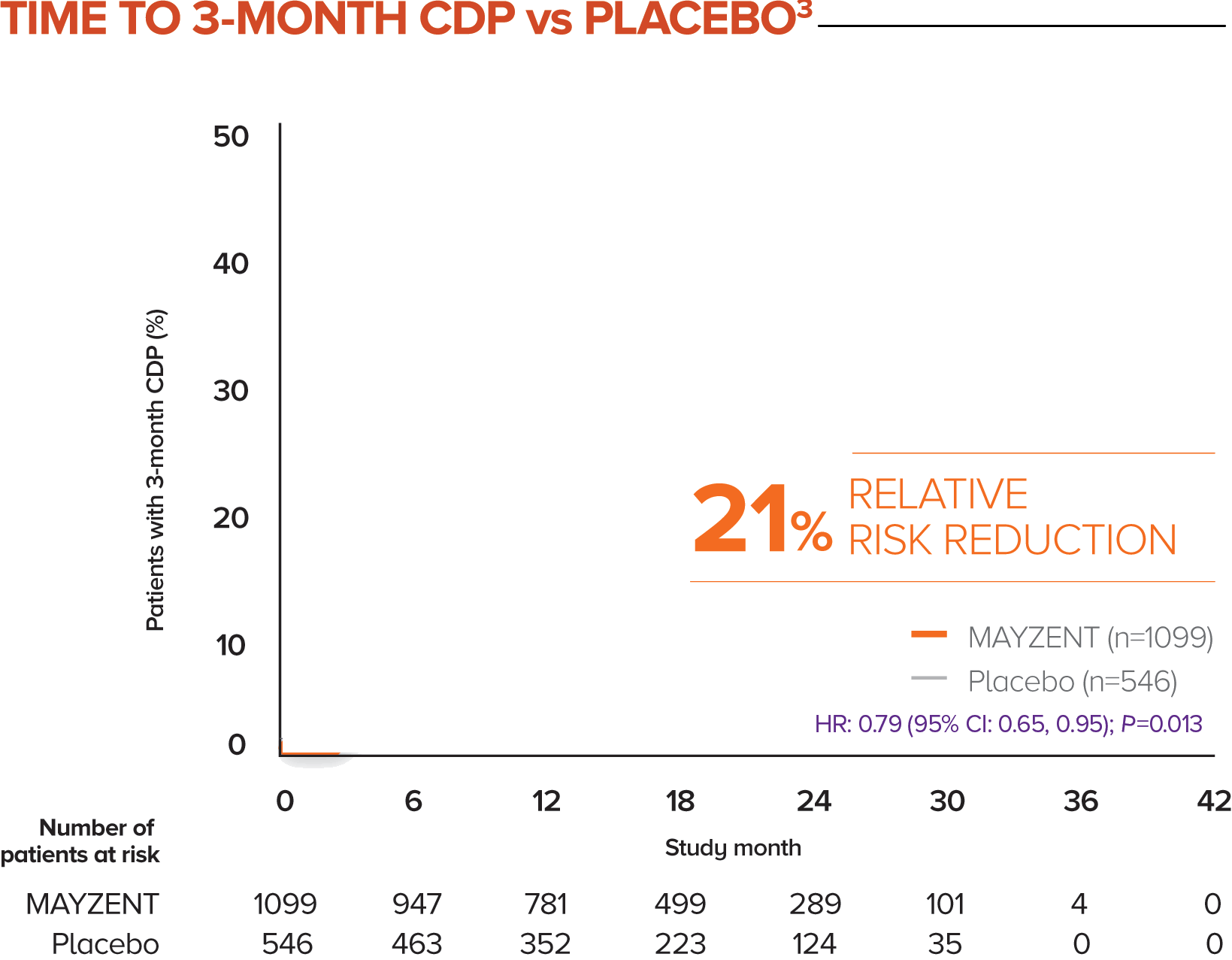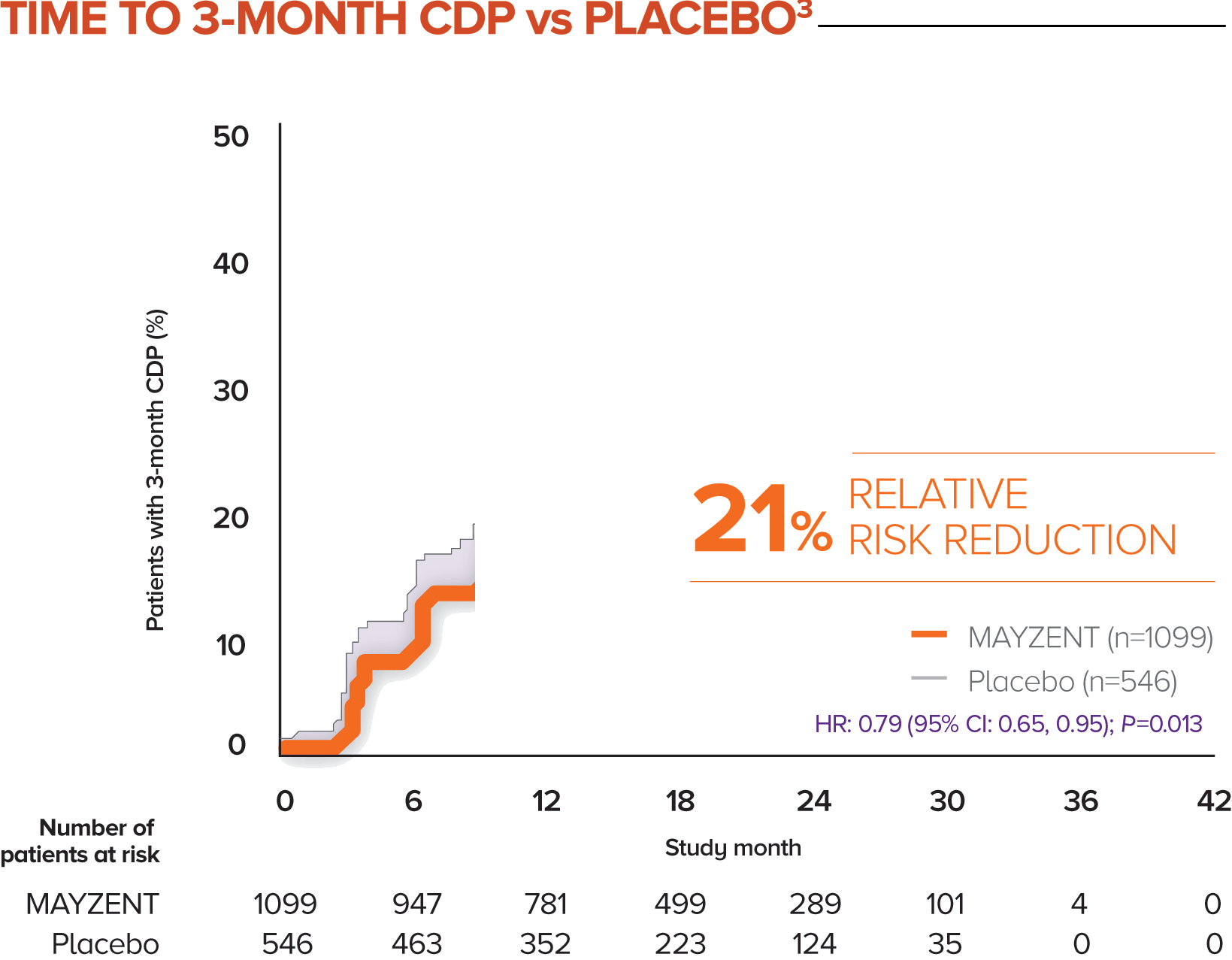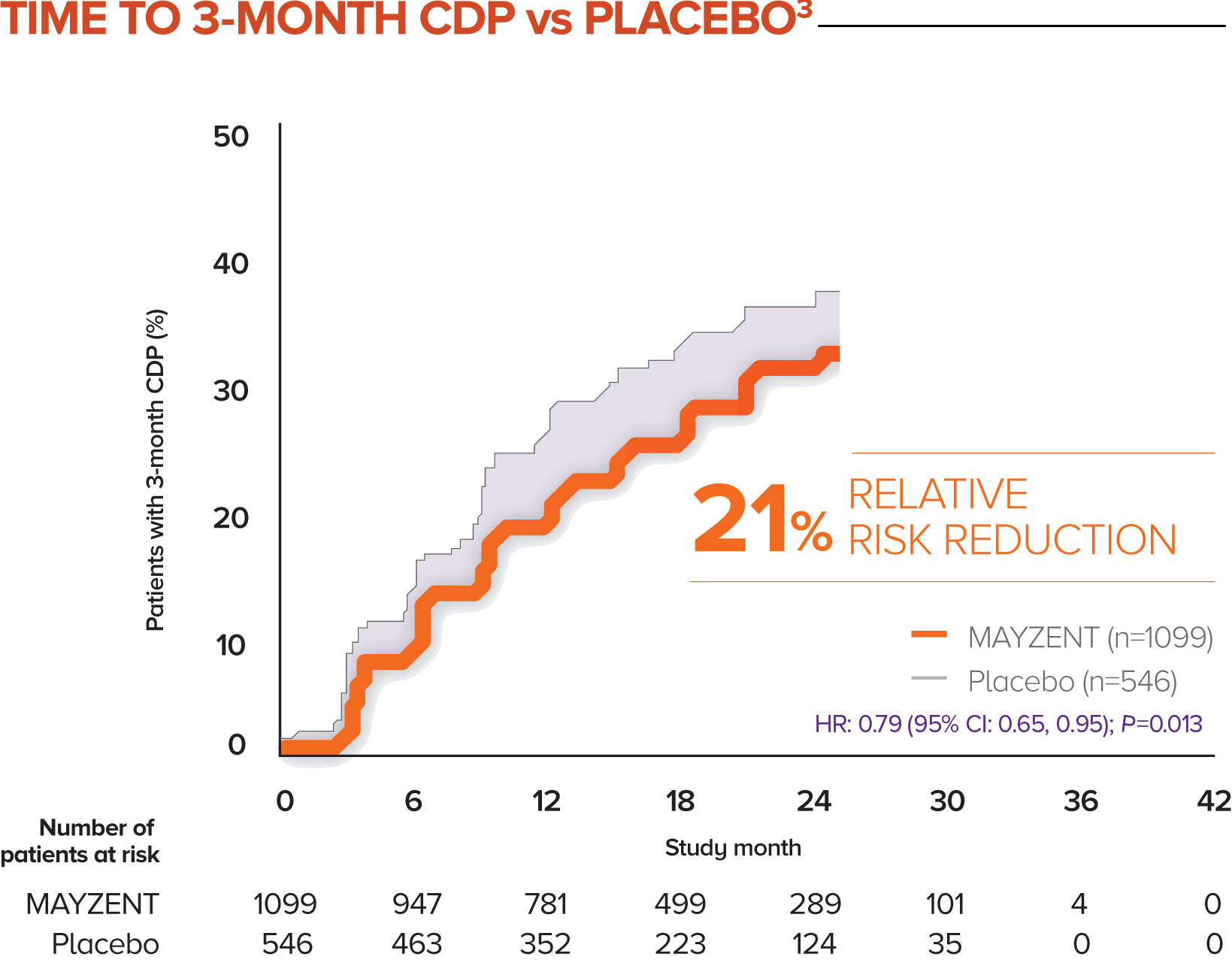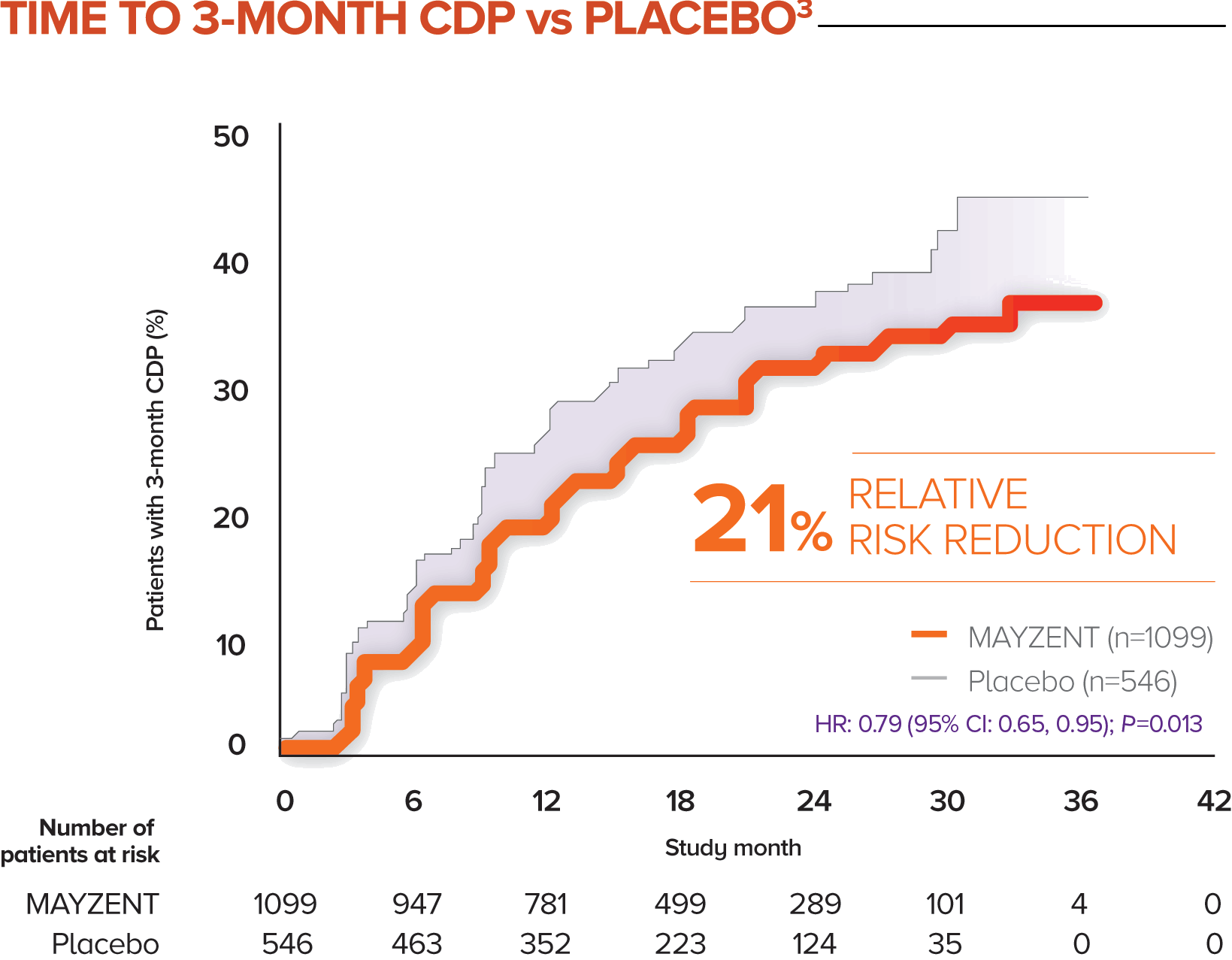
MAYZENT demonstrated a 21% relative risk reduction in time to 3-month CDP1,3
-
The proportion of patients with 3-month CDP for MAYZENT was 26% vs 32% for placebo1
-
CDP was defined as a ≥1-point increase from baseline in EDSS score (0.5-point increase for patients with a baseline EDSS score of ≥5.5) sustained for 3 months3
-
Although MAYZENT had a significant effect on CDP in patients with active SPMS (relapse in the 2 years prior to study entry), its effect in patients with nonactive SPMS was not statistically significant3
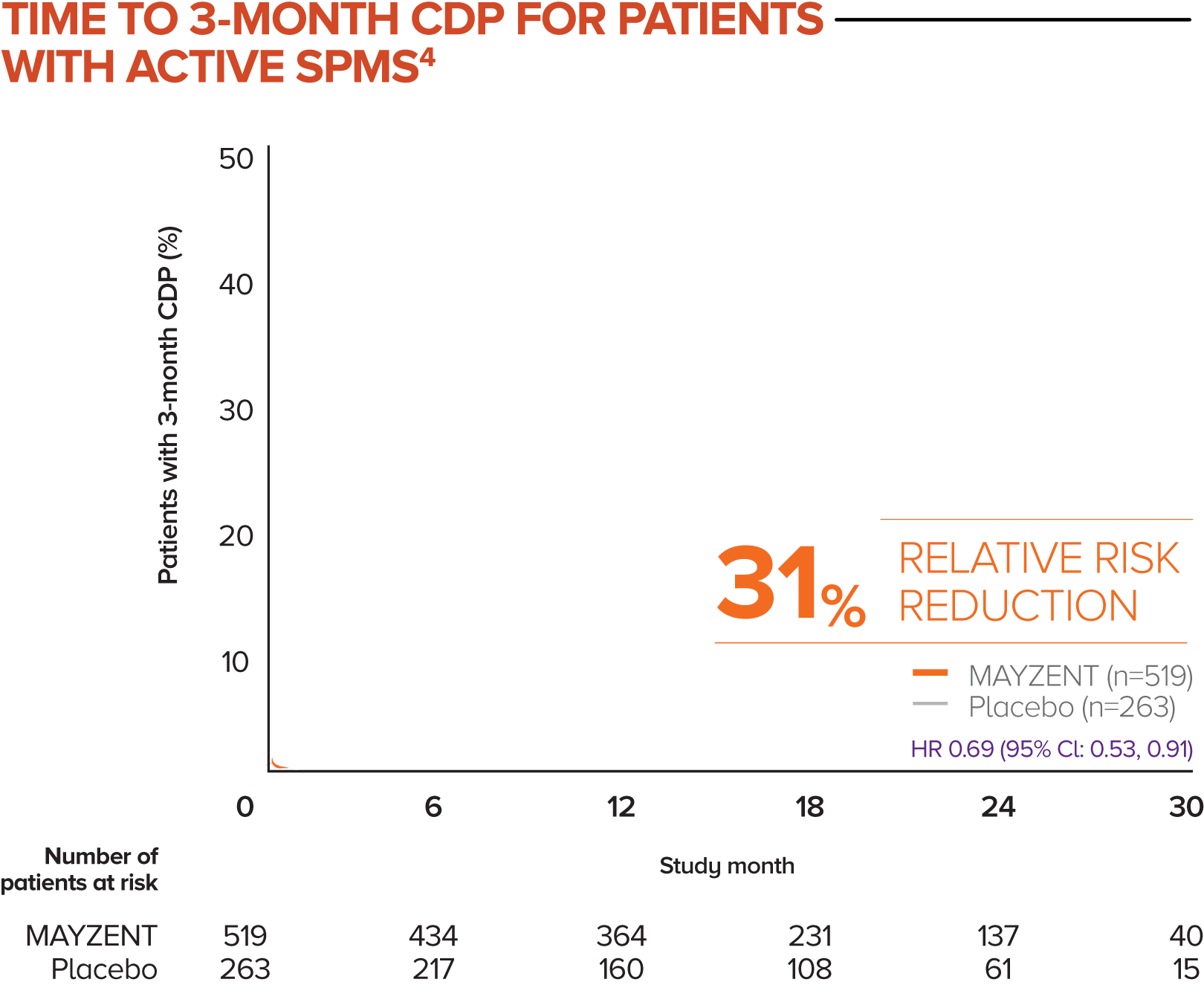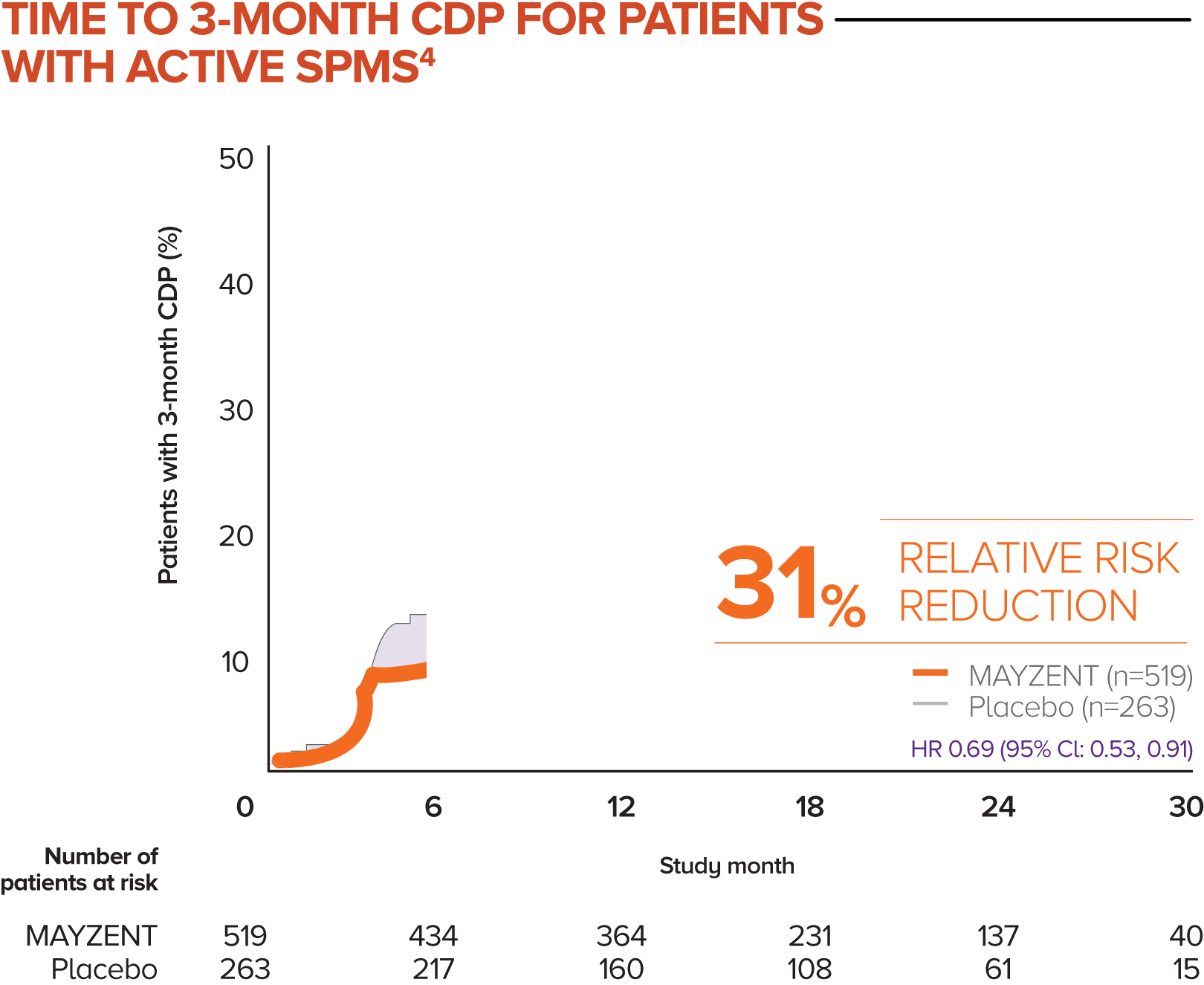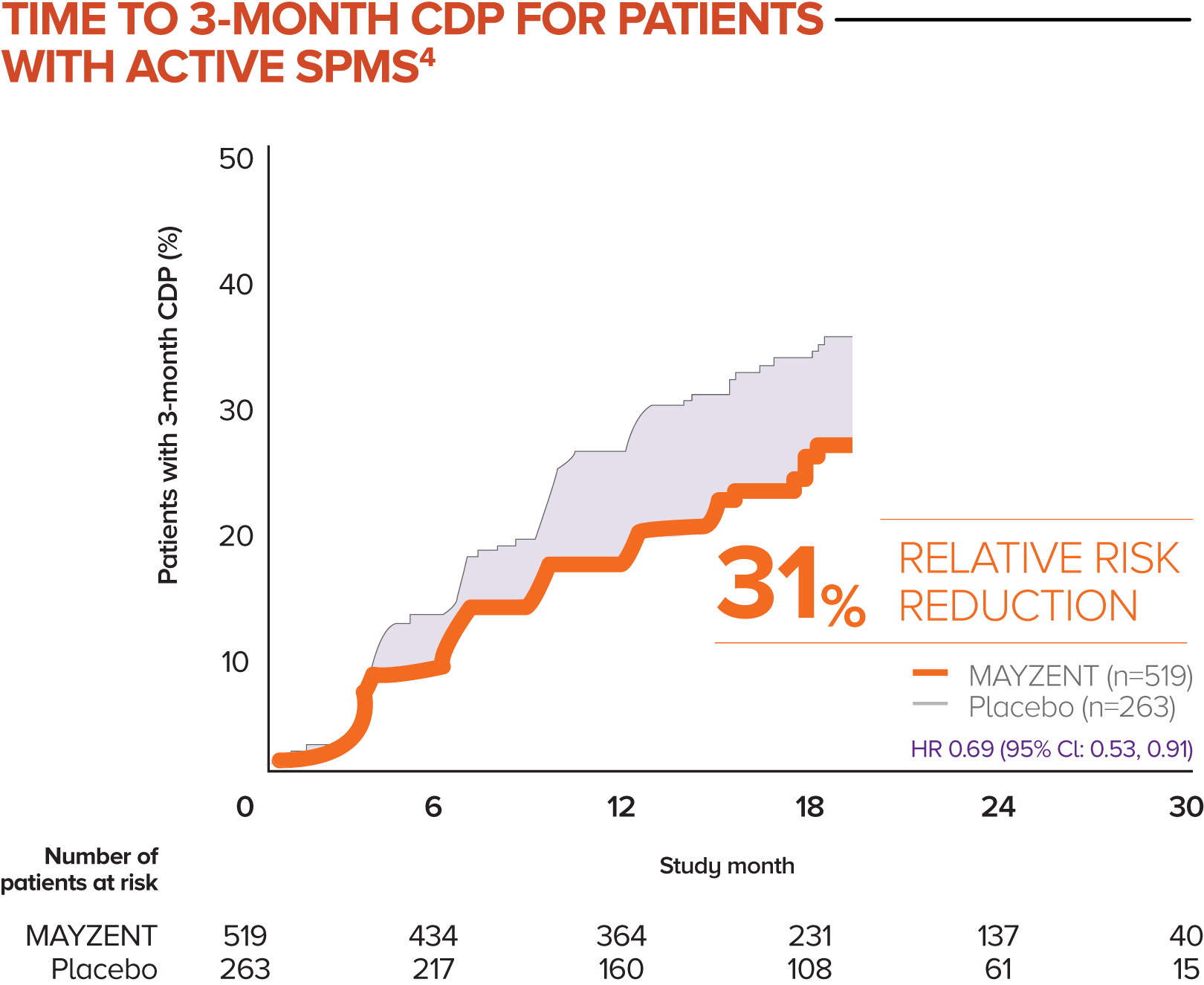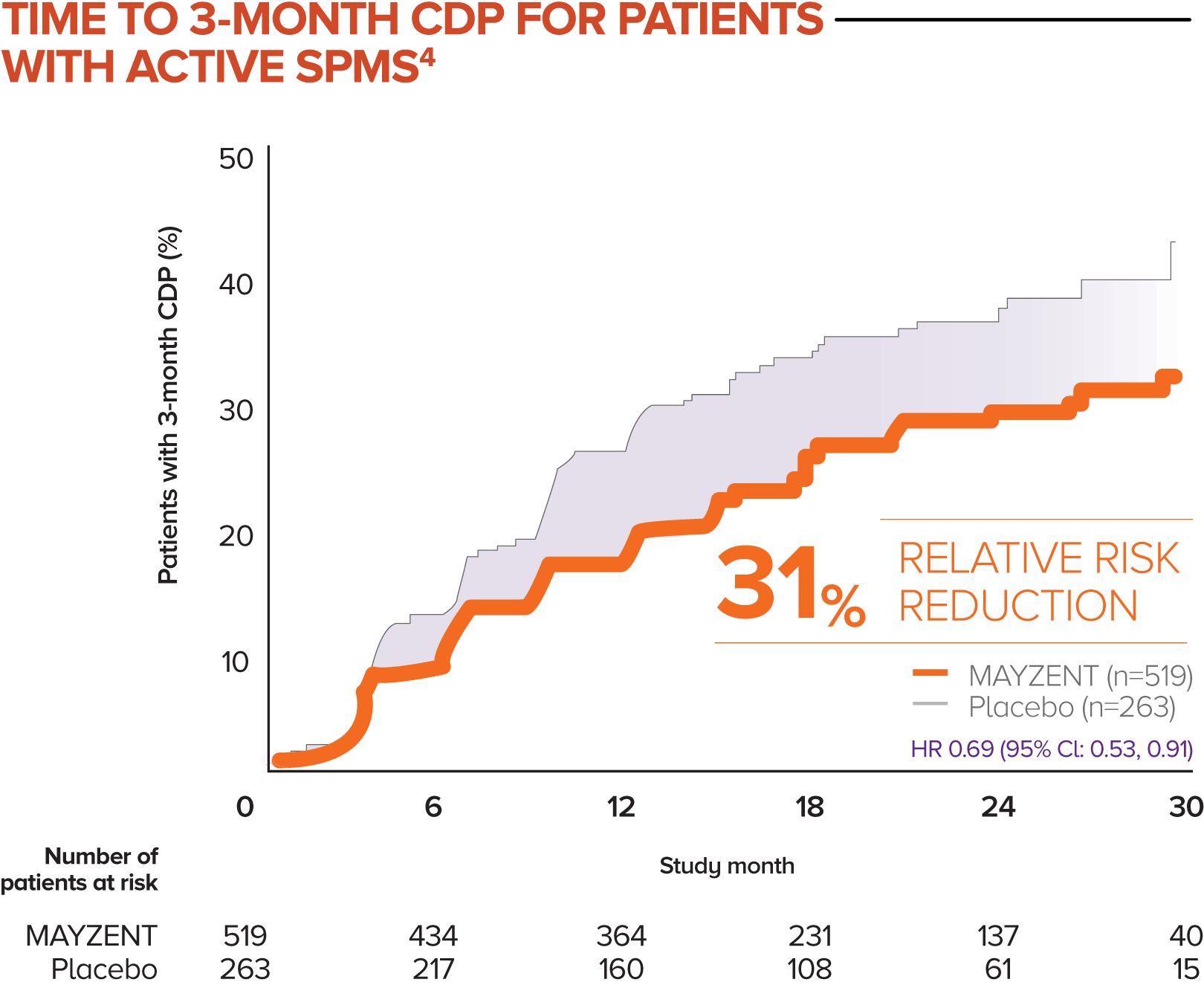
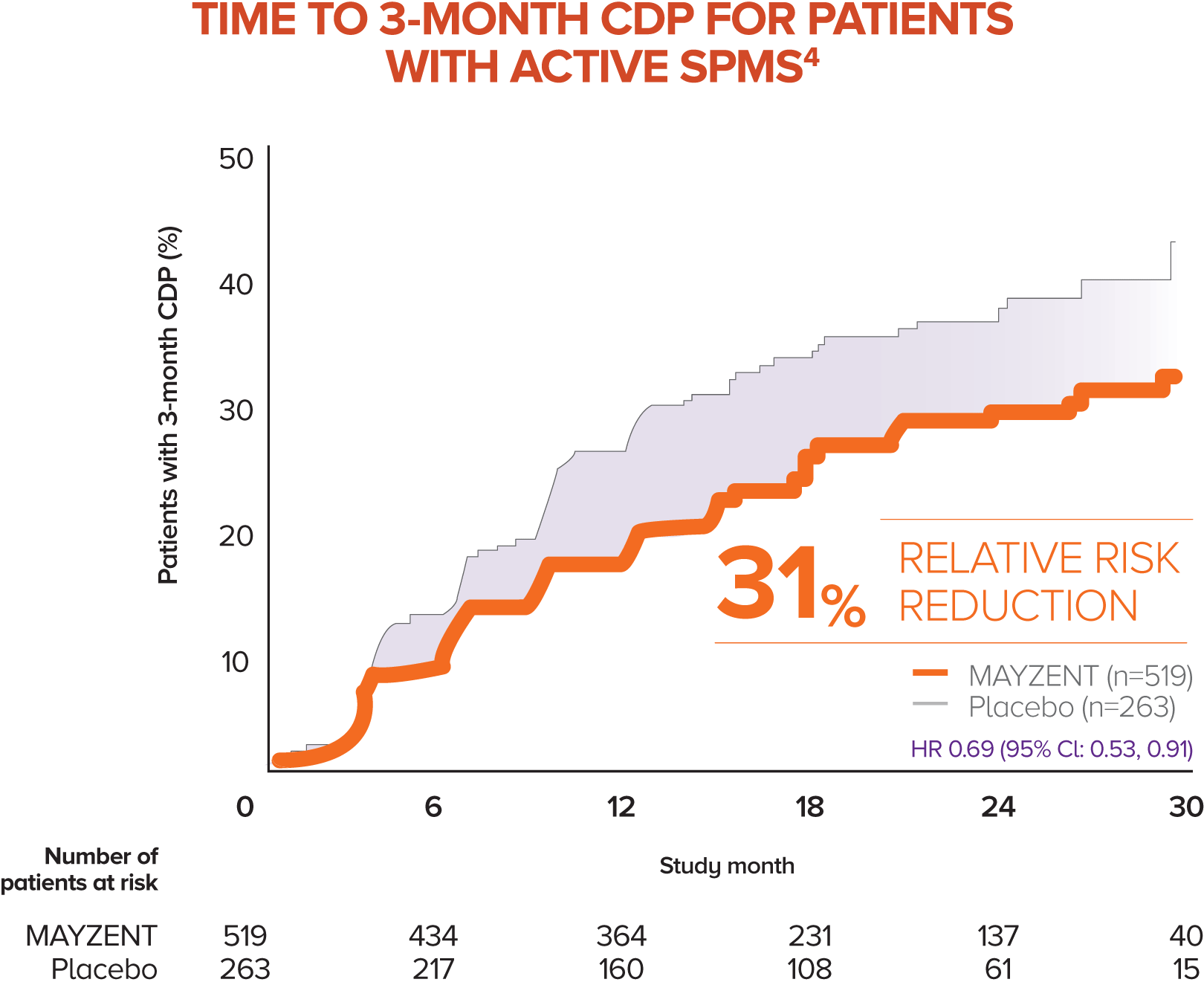
MAYZENT showed a 31% relative risk reduction in time to 3-month CDP vs placebo4
-
Active SPMS was defined as the presence of relapses in the 2 years prior to screening and/or ≥1 T1 GdE lesions at baseline
-
The separate analysis evaluated the efficacy of MAYZENT in delaying disability progression (time to 3-month CDP) in a subgroup of patients showing progression in RMS (active SPMS)
-
The proportion of patients with 3-month CDP for MAYZENT was 25% vs 35% for placebo
PATIENTS WITH DISABILITY IN EXPAND
56% of patients required walking assistance at
baseline in EXPAND. Therefore, upon study entry,
more than half of the patients in this trial relied on
a walker or cane to get around1,5
PATIENTS WITH DISABILITY IN EXPAND
56% of patients required walking assistance at baseline in EXPAND. Therefore, upon study entry, more than half of the patients in this trial relied on a walker or cane to get around1,5
MAYZENT WAS STUDIED IN SPMS PATIENTS WITH MODERATE-TO-ADVANCED DISABILITY ACROSS A BROAD RANGE OF END POINTS1
Key secondary end point results3*
T25-FW TEST
Time to 3-month confirmed deterioration by ≥20% on the T25-FW test was not statistically significant vs placebo (P=NS)
CNS TISSUE MEASURE
T2 LESION VOLUME
Reduced the expansion of T2 lesion volume at 12 and 24 months vs placebo (adjusted mean; P<0.01†)
-
Change from baseline in T2 lesion volume: 184 mm3 for patients on MAYZENT vs 879 mm3 for placebo
†Nominal P value, not corrected for multiple comparisons.
-
In EXPAND, a prespecified hierarchical analysis consisted of the primary end point and these 2 key secondary end points
-
The T25-FW test key end point was not significant; therefore, the T2 lesion volume key secondary end point was considered nominal
-
The remaining end points were not corrected for multiple comparisons.1,3
Additional secondary end point results1,3
ANNUALIZED RELAPSE RATE
55% relative reduction in ARR vs placebo
-
Defined as the average number of confirmed relapses per year (0.071 for MAYZENT vs 0.160 for placebo)
CNS TISSUE MEASURE:
NEW OR ENLARGING
T2 LESIONS
81% relative reduction in the mean number of new or enlarging T2 lesions (0.70 for patients on MAYZENT vs 3.60 for placebo) over all visits
6-MONTH CDP
26% relative risk reduction in disability progression as measured by time to 6-month CDP vs placebo
-
The proportion of patients with 6-month CDP for MAYZENT was 20% vs 26% for placebo
CNS TISSUE MEASURE:
T1 GdE LESIONS
86% relative reduction in the number of T1 GdE lesions on an MRI at 2 years for patients taking MAYZENT vs placebo (0.08 for patients on MAYZENT vs 0.60 for placebo)
CNS TISSUE MEASURE:
BRAIN VOLUME
LOSS
23% relative reduction in brain volume loss vs placebo
-
Change in brain volume from baseline was evaluated in 1342 patients (903 MAYZENT vs 439 placebo) at Month 12 and in 709 patients (470 MAYZENT vs 239 placebo) at Month 24 (adjusted mean percentage change from baseline was 0.50% for patients on MAYZENT vs 0.65% for placebo)
These analyses have not been adjusted for multiple comparisons. No conclusions of statistical or clinical significance can be drawn.
Additional secondary end point results1,3
ANNUALIZED RELAPSE RATE
55% relative reduction in ARR vs placebo
-
Defined as the average number of confirmed relapses per year (0.071 for MAYZENT vs 0.160 for placebo)
6-MONTH CDP
26% relative risk reduction in disability progression as measured by time to 6-month CDP vs placebo
-
The proportion of patients with 6-month CDP for MAYZENT was 20% vs 26% for placebo
CNS TISSUE MEASURE:
NEW OR ENLARGING
T2 LESIONS
81% relative reduction in the mean number of new or enlarging T2 lesions (0.70 for patients on MAYZENT vs 3.60 for placebo) over all visits
CNS TISSUE MEASURE:
T1 GdE LESIONS
86% relative reduction in the number of T1 GdE lesions on an MRI at 2 years for patients taking MAYZENT vs placebo (0.08 for patients on MAYZENT vs 0.60 for placebo)
CNS TISSUE MEASURE:
BRAIN VOLUME LOSS
23% relative reduction in brain volume loss vs placebo
-
Change in brain volume from baseline was evaluated in 1342 patients (903 MAYZENT vs 439 placebo) at Month 12 and in 709 patients (470 MAYZENT vs 239 placebo) at Month 24 (adjusted mean percentage change from baseline was 0.50% for patients on MAYZENT vs 0.65% for placebo)
These analyses have not been adjusted for multiple comparisons. No conclusions of statistical or clinical significance can be drawn.
Exploratory cognitive end point results6-9

3 COGNITIVE TESTS
SDMT is a written and oral test for assessing cognitive processing speed—the most frequently affected cognitive domain in progressing patients. The test involves both rapid information processing and attention skills.‡
-
21.3% overall reduction in the risk of sustained decrease in SDMT score of ≥4 points for patients on MAYZENT (a 4-point SDMT change is regarded as a clinically meaningful change)
-
2.48-point difference vs placebo at 24 months (adjusted mean)
The results from PASAT and BVMT-R showed no clinically meaningful difference between MAYZENT and placebo.§
These analyses have not been adjusted for multiple comparisons. No conclusions of statistical or clinical significance can be drawn.
Post hoc analysis results from an EXPAND substudy on cortical and thalamic grey matter volume10
Cortical grey matter and thalamic grey matter volume were assessed in a post hoc analysis of pooled data from EXPAND. The dataset included patients who participated in a prospective high-resolution T1 MRI substudy and a post hoc analysis of patients who underwent conventional MRI. The full analysis set (no exclusions applied) and the per-protocol set (excluding patients with major protocol deviations and efficacy data after discontinuation) were assessed.10
PER-PROTOCOL SET ANALYSIS10,11:
CNS TISSUE MEASURE:
GREY MATTER VOLUME LOSS

CNS TISSUE MEASURE:
GREY MATTER VOLUME LOSS

63% RELATIVE REDUCTION
in cortical grey matter volume loss
vs placebo in 1029 patients (692 MAYZENT vs 337 placebo) at Month 24 (adjusted mean percentage change from baseline was -0.39 for patients on MAYZENT vs -1.04 for placebo)
42% RELATIVE REDUCTION
in thalamic grey matter volume loss
vs placebo in 1038 patients (696 MAYZENT vs 342 placebo) at Month 24 (adjusted mean percentage change from baseline was -1.02 for patients on MAYZENT vs -1.77 for placebo)
The full analysis set of MAYZENT vs placebo showed a relative reduction of 43% for cortical grey matter volume loss in 1315 patients (886 vs 429) and 31% for thalamic grey matter volume loss in 1329 patients (892 vs 437) at Month 24, respectively.10
These analyses have not been adjusted for multiple comparisons. No conclusions of statistical or clinical significance can be drawn.
POST HOC SUBGROUP ANALYSIS:
Progression to wheelchair use was assessed in the most progressed patients in EXPAND1,12POST HOC SUBGROUP ANALYSIS:
Progression to wheelchair use was assessed in the most progressed patients in EXPAND1,12-
The analysis evaluated the efficacy of MAYZENT in delaying disability progression in patients with an EDSS score of 6.5 (at baseline) to an EDSS score of ≥7, throughout the trial period12||
-
The EDSS equates a score of 6.5 with the requirement of assistance to walk (eg, walker), and a score of 7.0 with the use of a wheelchair5



MAYZENT reduced the risk of progressing from an EDSS score of 6.5 to ≥7 by 37% vs placebo

MAYZENT reduced the risk of progressing from an EDSS score of 6.5 to ≥7 by 37% vs placebo
The proportion of patients with an EDSS score of 6.5 (at baseline) to ≥7 (at trial’s end) was 19.2% for MAYZENT vs 26.1% for placebo.
These analyses have not been adjusted for multiple comparisons. No conclusions of statistical or clinical significance can be drawn.
EXPAND OPEN-LABEL
EXTENSION STUDY INTERIM ANALYSIS OF SAFETY AND EXPLORATORY DATA1,2
SELECT EFFICACY ASSESSMENTS UP TO 5 YEARS WERE CONSISTENT WITH THE CORE STUDY1,2¶
OPEN-LABEL EXTENSION INTERIM ANALYSIS
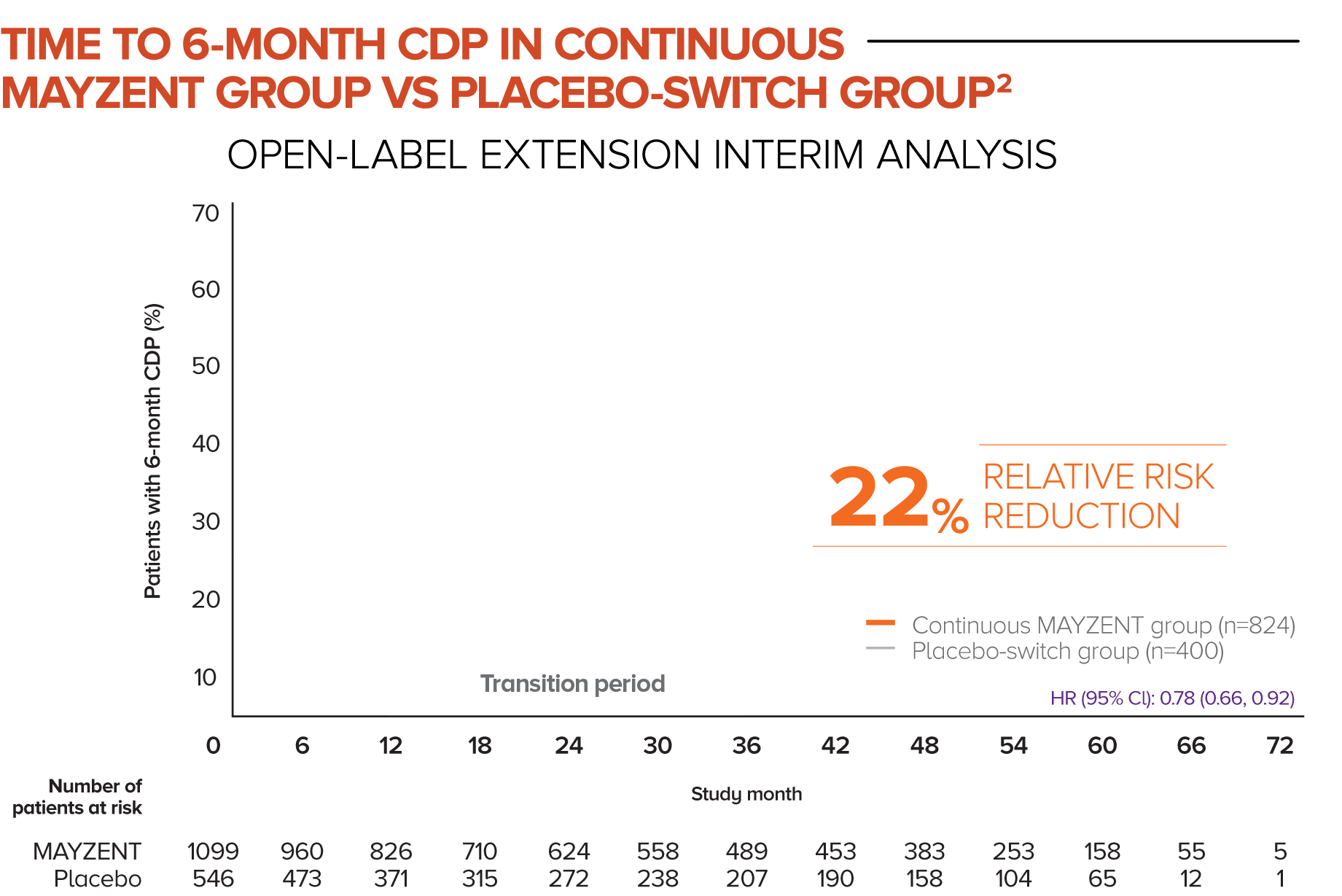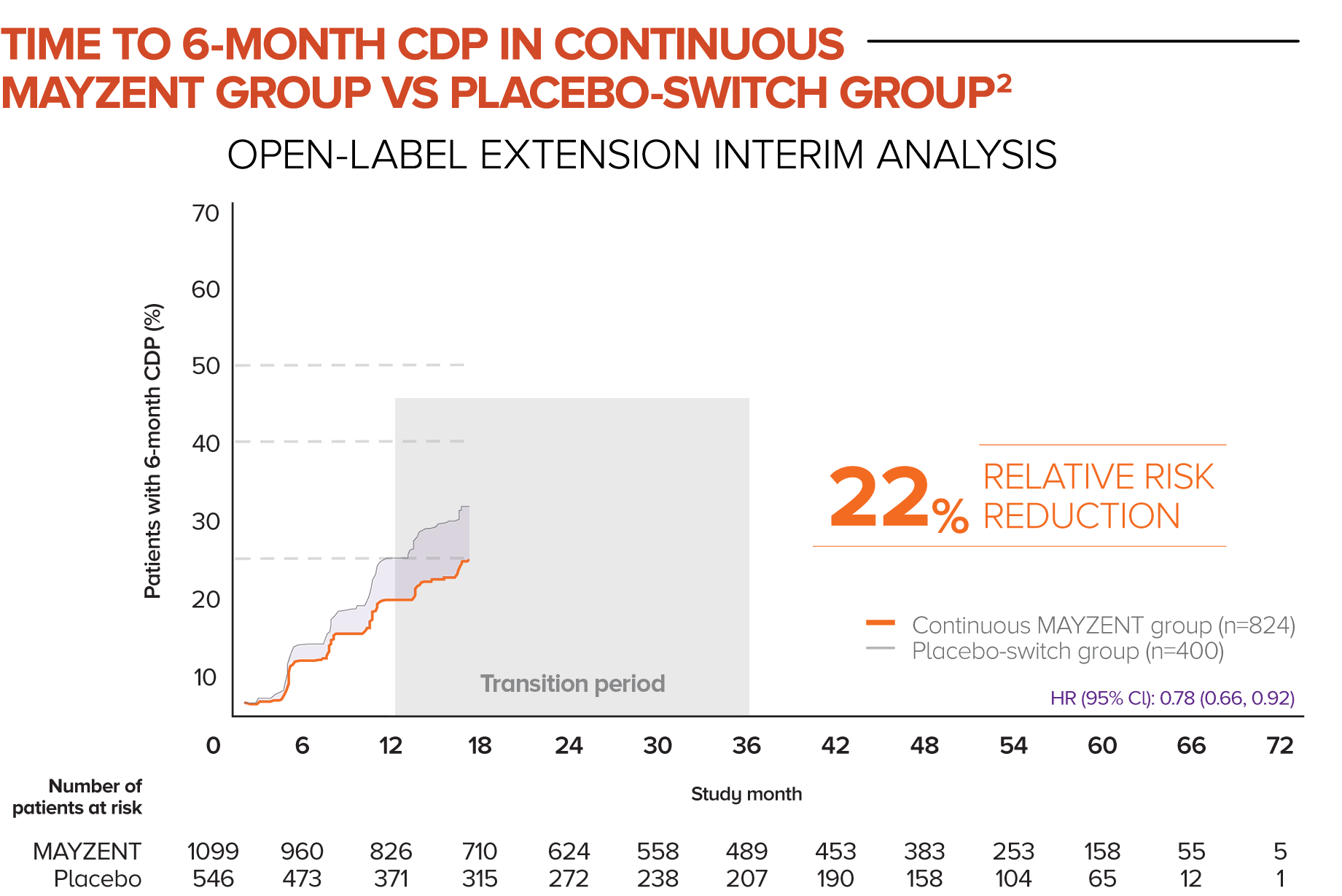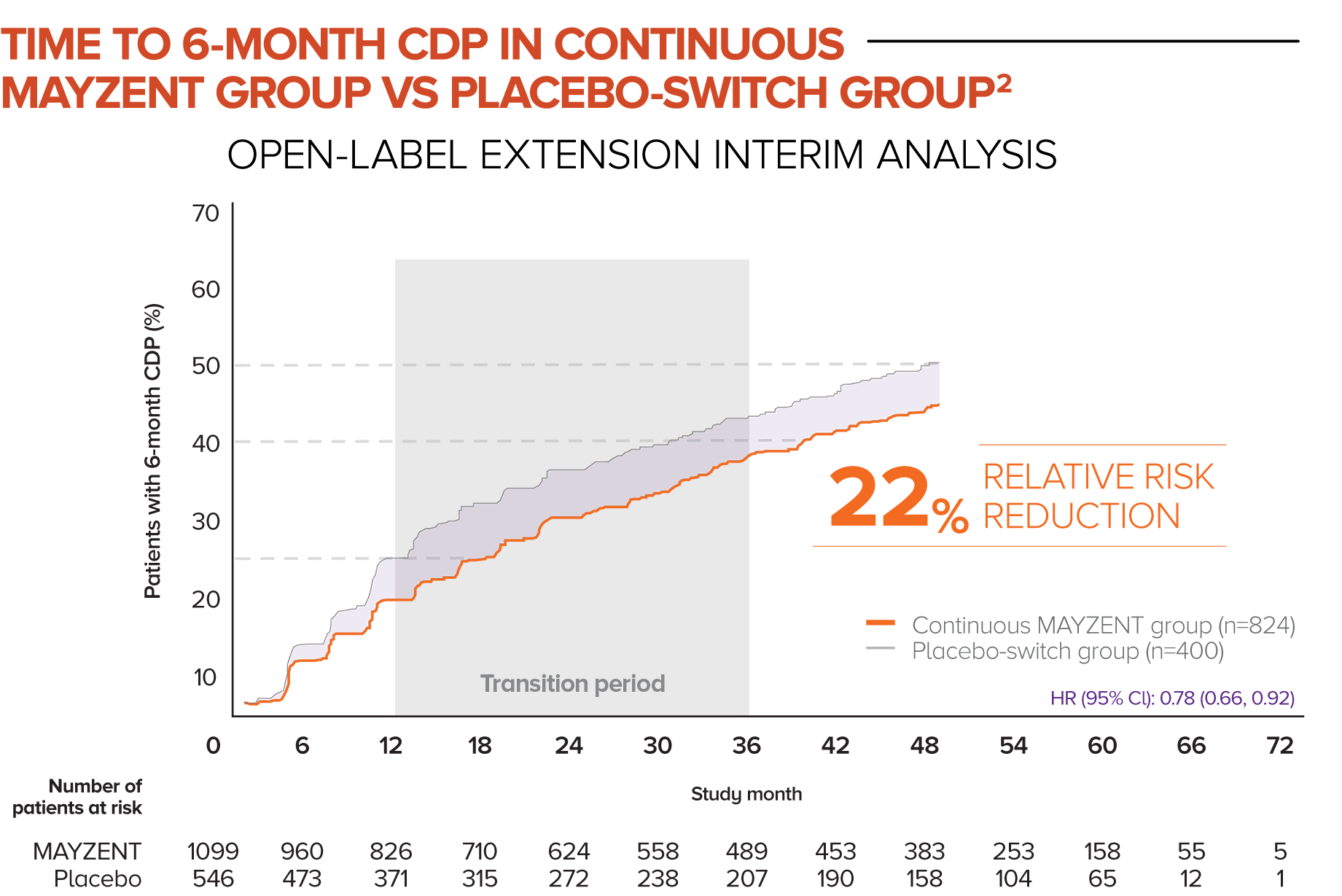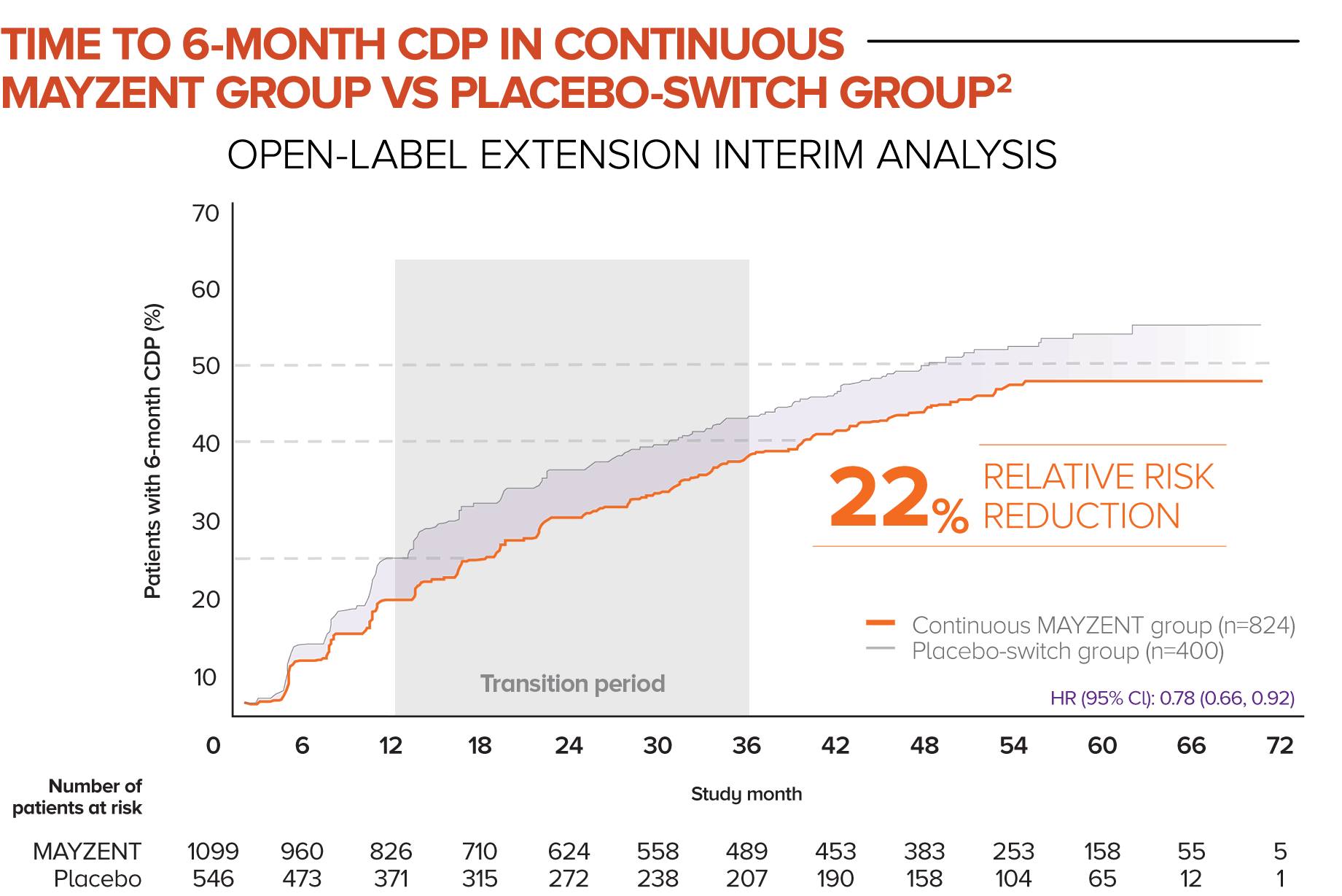
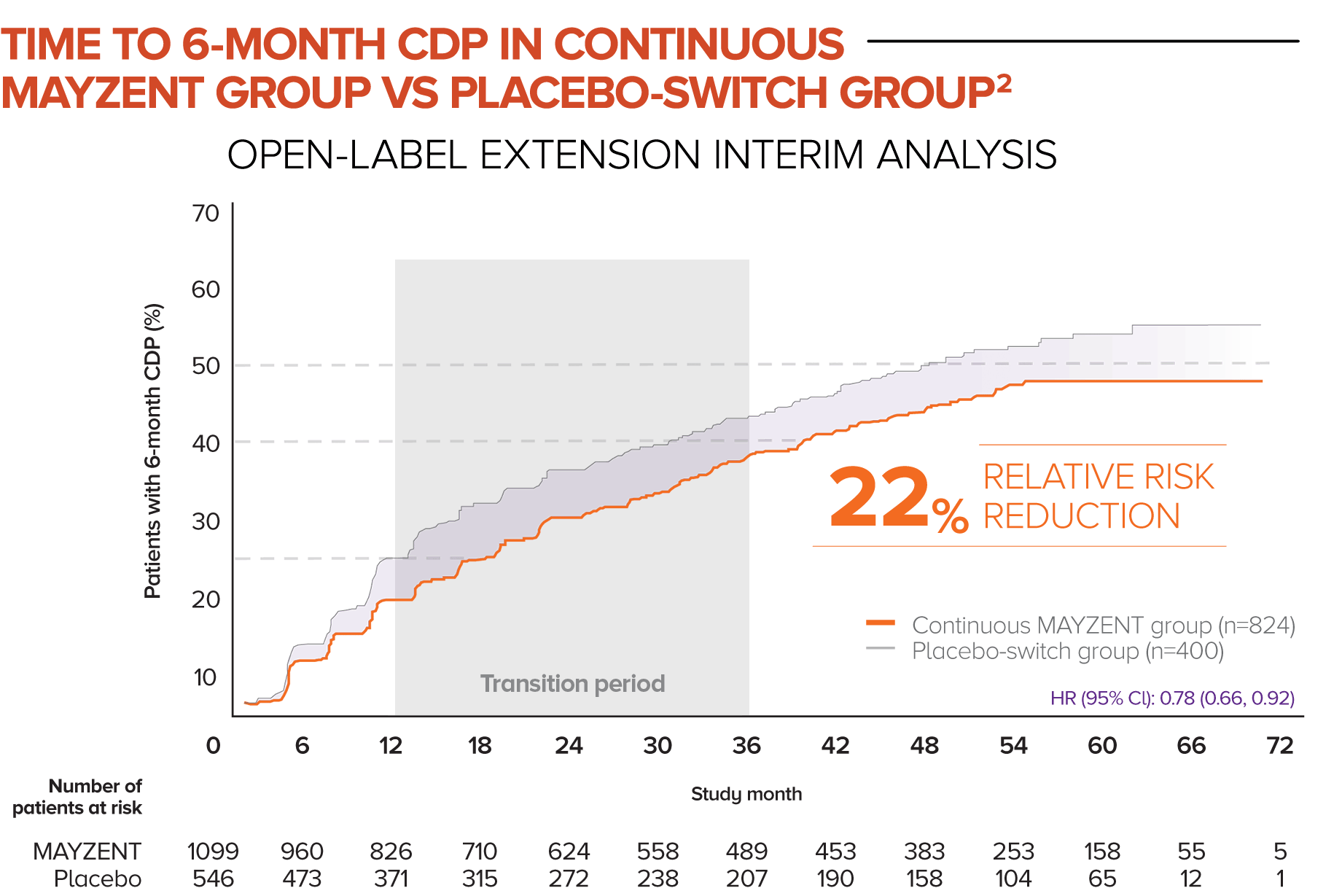
In this analysis, patients who started in the MAYZENT treatment arm experienced a greater reduction in the risk of disability progression vs patients who switched to MAYZENT later
STUDY DESIGNANNUALIZED RELAPSE RATE
52% RELATIVE REDUCTION in ARR vs placebo-switch group
-
Defined as the average number of confirmed relapses per year (0.051 for MAYZENT vs 0.106 for placebo-switch group)
-
In this analysis, patients who started treatment on MAYZENT earlier saw a reduction in ARR vs patients who switched to MAYZENT later from placebo
COGNITIVE PROCESSING SPEED
23% OVERALL REDUCTION in the risk of decrease in SDMT score vs placebo-switch group
-
SDMT was the only cognitive assessment conducted in the extension study
The extension study end points differ from the core study and were predefined as exploratory in the extension protocol.13
These exploratory analyses represent chance findings. No conclusions of statistical or clinical significance can be drawn. Consider interim analysis open-label extension study limitations when interpreting data. The open-label extension study is not blinded, not controlled, and includes inherent self-selection bias for remaining in the trial.
SDMT was assessed but was not an exploratory end point in the extension study. Additional long-term data that was collected but not listed: 6-month confirmed worsening of at least 20% from baseline in the T25-FW test, MRI parameters, Multiple Sclerosis Walking Scale-12, and EuroQoL.2,13
¶6-month CDP, ARR, and SDMT were exploratory end points and assessments of efficacy measurements, respectively, in the EXPAND extension study.13
GET PATIENTS STARTED ON MAYZENT®
ARR, annualized relapse rate; BVMT-R, Brief Visuospatial Memory Test Revised; CDP, confirmed disability progression; CI, confidence interval; CNS, central nervous system; EDSS, Expanded Disability Status Scale; GdE, gadolinium-enhancing; HR, hazard ratio; MOA, mechanism of action; MOD, mechanism of disease; MRI, magnetic resonance imaging; MS, multiple sclerosis; NS, not significant; PASAT, Paced Auditory Serial Addition Test; RMS, relapsing MS; SDMT, Symbol Digit Modalities Test; SPMS, secondary progressive MS; T25-FW, timed 25-foot walk.
‡Results for SDMT oral scores reported at Months 12 and 24.8
§PASAT is a test that assesses auditory information processing speed and flexibility; BVMT-R is an assessment of visuospatial memory.9
||Measured the length of time until a person reached an EDSS score of ≥7 based on sustained progression until the end of the EXPAND core phase. The survival analysis included a subset of patients with a baseline EDSS score of 6.5. Time to sustained progression to an EDSS score of ≥7 was assessed by a Cox proportional hazards model, with treatment as the covariate. The proportion of patients with sustained progression to an EDSS score of ≥7 was analyzed by Kaplan-Meier curves. Analyses excluded all EDSS assessments that were conducted during relapse. The adjusted/weighted survival analysis was performed in the overall patient population.12
References: 1. Kappos L, Bar-Or A, Cree BAC, et al; for the EXPAND Clinical Investigators. Siponimod versus placebo in secondary progressive multiple sclerosis (EXPAND): a double-blind, randomised, phase 3 study. Lancet. 2018;391(10127):1263-1273. 2. Data on file. Long-term Efficacy and Safety of Siponimod in Patients with SPMS: EXPAND Extension Analysis up to 5 Years. Novartis Pharmaceuticals Corp; May 2020. 3. Mayzent [prescribing information]. East Hanover, NJ: Novartis Pharmaceuticals Corp. 4. Data on file. Efficacy of Siponimod in Secondary Progressive Multiple Sclerosis Patients With Active Disease: The EXPAND Study Subgroup Analysis. Novartis Pharmaceuticals Corp; August 2019. 5. Kurtzke JF. Rating neurologic impairment in multiple sclerosis: an expanded disability status scale (EDSS). Neurology. 1983;33(11):1444-1452. 6. Data on file. Impact of Siponimod on Cognition in Patients With Secondary Progressive Multiple Sclerosis: Results From Phase 3 EXPAND Study. Novartis Pharmaceuticals Corp; 2017. 7. Benedict RH, DeLuca J, Phillips G, et al. Validity of the Symbol Digit Modalities Test as a cognition performance outcome measure for multiple sclerosis. Mult Scler. 2017;23(5):721-733. 8. Data on file. Benedict AAN. Novartis Pharmaceuticals Corp; April 2018. 9. Data on file. Benedict Cognition Data. Novartis Pharmaceuticals Corp; 2017. 10. Data on file. Effect of Siponimod on Cortical Grey Matter and Thalamic Volume in Patients With Secondary Progressive Multiple Sclerosis–Results of the EXPAND Study. Novartis Pharmaceuticals Corp; May 2020. 11. Data on file. Siponimod Reduces Grey Matter Atrophy in Patients With Secondary Progressive Multiple Sclerosis: Subgroup Analyses From the EXPAND Study. Novartis Pharmaceuticals Corp; May 2020. 12. Data on file. Data Analysis Report Time to Wheelchair. Novartis Pharmaceuticals Corp; September 2019. 13. Data on file. A multicenter, randomized, double-blind, parallel-group, placebo-controlled variable treatment duration study evaluating the efficacy and safety of siponimod (BAF312) in patients with secondary progressive multiple sclerosis followed by extended treatment with open-label BAF312. Novartis Pharmaceuticals Corp; July 2020.