THE SAFETY PROFILE OF MAYZENT® REMAINED CONSISTENT WITH THE CORE STUDY UP TO 5 YEARS1

EXPAND CORE SAFETY2
-
Adverse events that occurred in ≥5% of patients taking MAYZENT, and
-
Occurred at a rate ≥1% higher than in patients receiving placebo
PROPORTION OF PATIENTS WITH ADVERSE EVENTS2

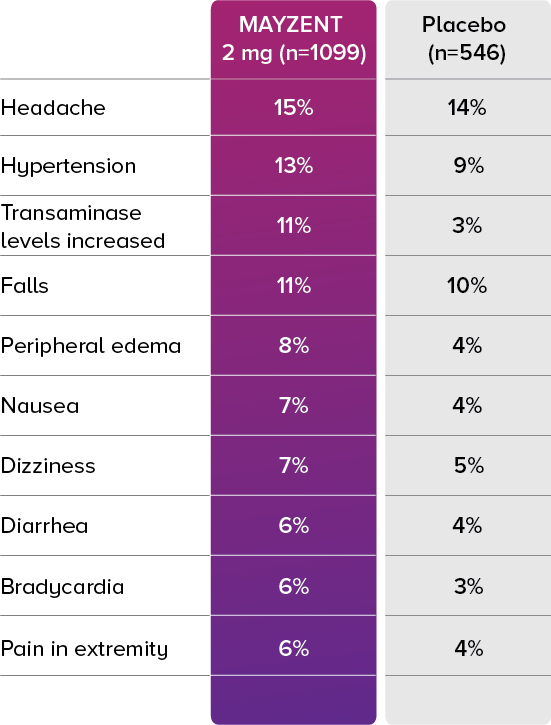
In the core study, the most common adverse events (incidence ≥10%) were headache (15%), hypertension (13%), and transaminase increases (11%).2
Treatment discontinuation rates due to adverse events were similar across treatment arms.
-
8.5% of patients taking MAYZENT discontinued treatment due to adverse events vs 5.1% with placebo2
In the core study, the most common adverse events (incidence ≥10%) were headache (15%), hypertension (13%), and transaminase increases (11%).2
Treatment discontinuation rates due to adverse events were similar across treatment arms.
-
8.5% of patients taking MAYZENT discontinued treatment due to adverse events vs 5.1% with placebo2
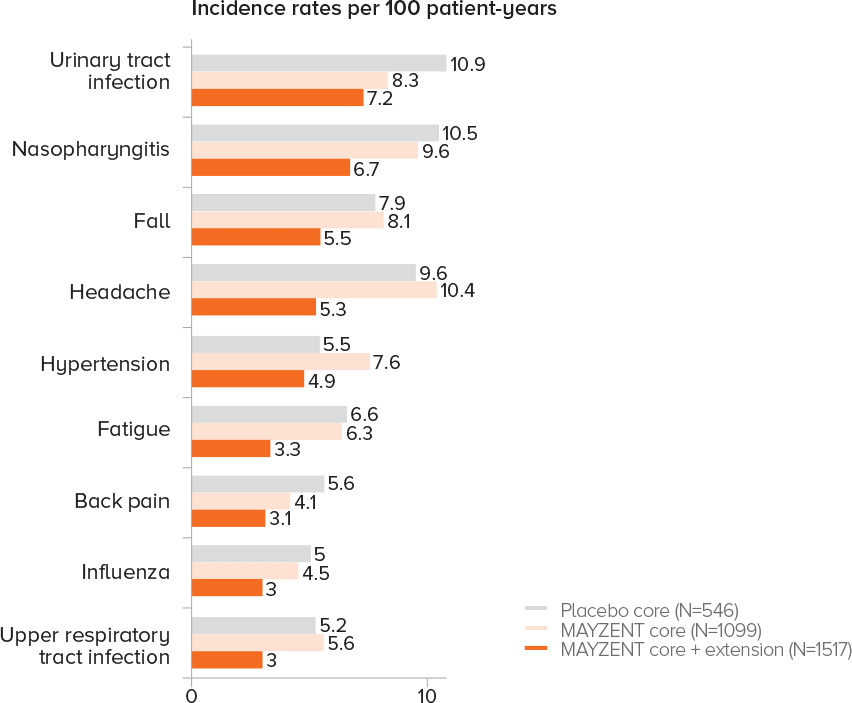
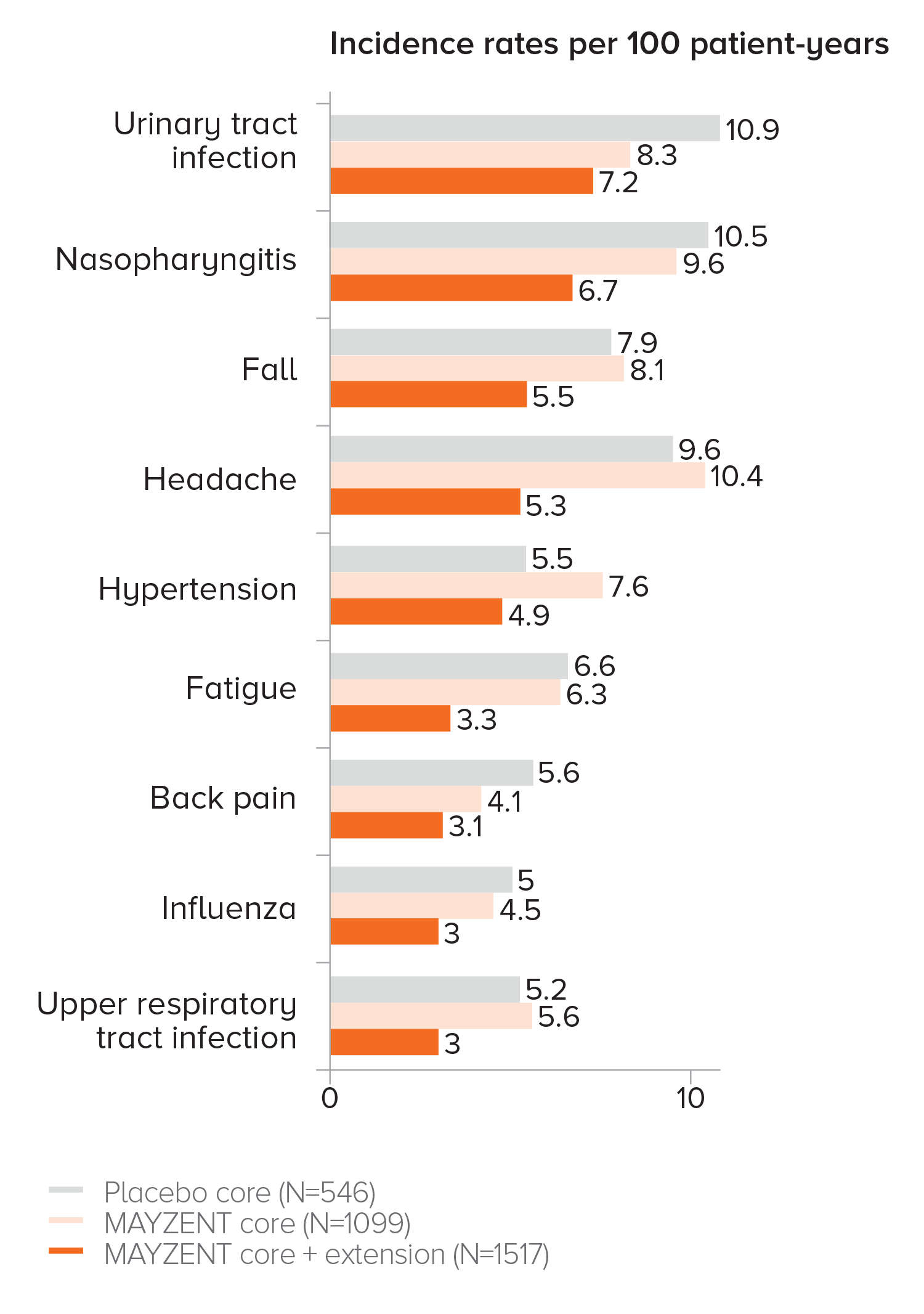
EXPAND EXTENSION STUDY SAFETY UP TO 5 YEARS1
-
Adverse events that occurred in ≥3% of patients taking MAYZENT in the core and extension studies
-
Analysis results were consistent with core study per listed criteria
PROPORTION OF PATIENTS WITH ADVERSE EVENTS1
Information at bottom of page.
Information at bottom of page.
CONSIDERATIONS FOR INITIATION2

Genotype testing: Patients undergo a genotype test to identify their specific variant of CYP2C9, the principal enzyme that metabolizes MAYZENT.* This genotype test identifies the appropriate MAYZENT maintenance dose.

Most patients won’t require an FDO during initiation: An FDO is required only for patients with certain preexisting cardiac conditions.2,3
Titration schedule: Initiation of MAYZENT treatment results in a transient decrease in heart rate and atrioventricular conduction delays. For all patients, a dose titration is required for initiation of MAYZENT treatment to help patients safely reach their maintenance dose.2
CONSIDERATIONS FOR INITIATION2
Genotype testing: Patients undergo a genotype test to identify their specific variant of CYP2C9, the principal enzyme that metabolizes MAYZENT.* This genotype test identifies the appropriate MAYZENT maintenance dose.
Most patients won’t require an FDO during initiation: An FDO is required only for patients with certain preexisting cardiac conditions.2,3
Titration schedule: Initiation of MAYZENT treatment results in a transient decrease in heart rate and atrioventricular conduction delays. For all patients, a dose titration is required for initiation of MAYZENT treatment to help patients safely reach their maintenance dose.2
GET PATIENTS STARTED ON MAYZENT®
FDO, first-dose observation; MOA, mechanism of action; MOD, mechanism of disease; MS, multiple sclerosis; RMS, relapsing MS.
*Patients with a CYP2C9*3/*3 genotype should not be treated with MAYZENT.2
*Patients with a CYP2C9*3/*3 genotype should not be treated with MAYZENT.2
References: 1. Data on file. Long-term Efficacy and Safety of Siponimod in Patients with SPMS: EXPAND Extension Analysis up to 5 Years. Novartis Pharmaceuticals Corp; May 2020. 2. Mayzent [prescribing information]. East Hanover, NJ: Novartis Pharmaceuticals Corp. 3. Data on file. Mayzent FDO Analysis. Novartis Pharmaceuticals Corp; March 2022.